Question
Question: The monomer of natural rubber is: A) 
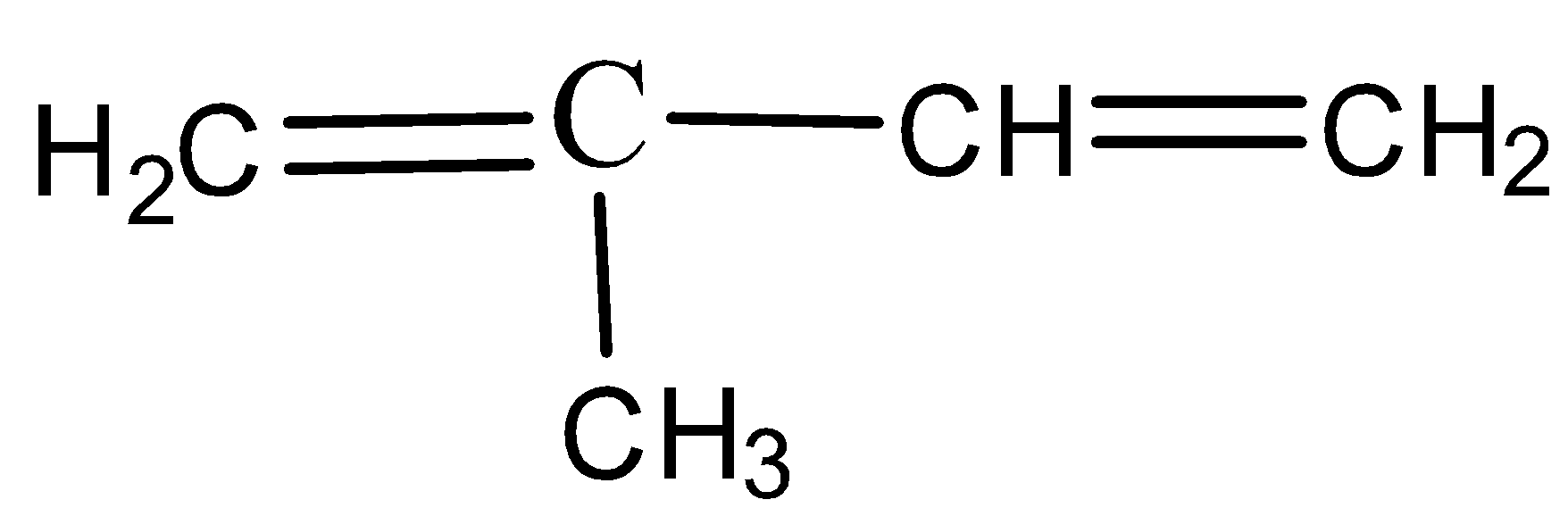
B)
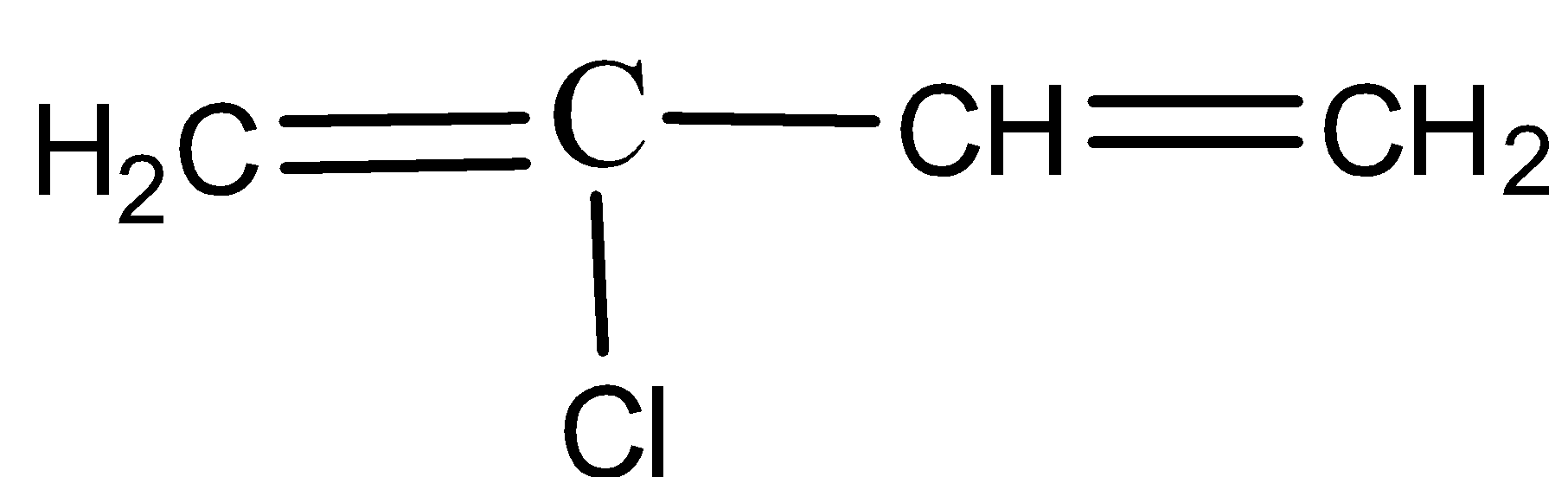
C)
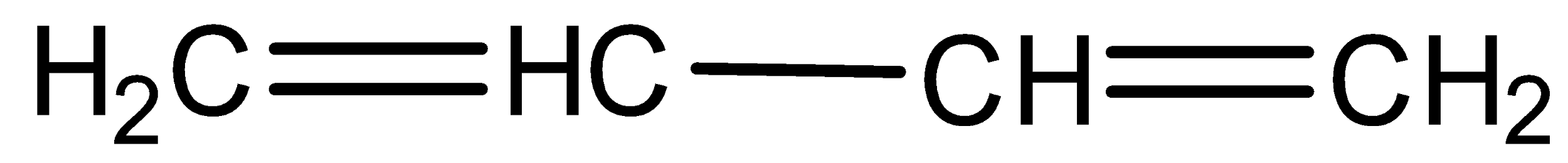
D) None of these
Solution
The answer to this question is based on the concept of the physical chemistry that includes the polymerisation topic where the monomer unit combines to form a polymer and this concept gives the answer.
Complete step by step answer:
We have come across the chapters in the physical chemistry classes which deal with the topic polymerisation and also several parameters related to it, that is, kinetics of polymerisation, types of polymerisation and also on.
We shall now see what does polymerisation mean and this can help us to deduce the required answer.
- Polymerisation is the process in which two or more monomer units combine to form long chain compounds that are the polymers.
- Polymers play an important role in our daily lives.
- Most of the things we use are made of polymer , for instance clothes, toys, comb, thread and many more.
- Therefore, polymers have a wide range of applications and thus are the important components of the industrial process.
- There are various types of polymerisation like addition polymerisation, condensation polymerisation, copolymerisation etc., that are based on the mechanism of polymerisation.
We shall see the monomer unit of rubber that is obtained naturally from the plants.
- Rubber is made up of small monomer units that are called as isoprene units. These isoprene units are made of unsaturated carbon atom with a methyl group attached to one of the four carbon atom and the IUPAC name is given by, 2-methyl 1,3-butadiene which is as shown below,
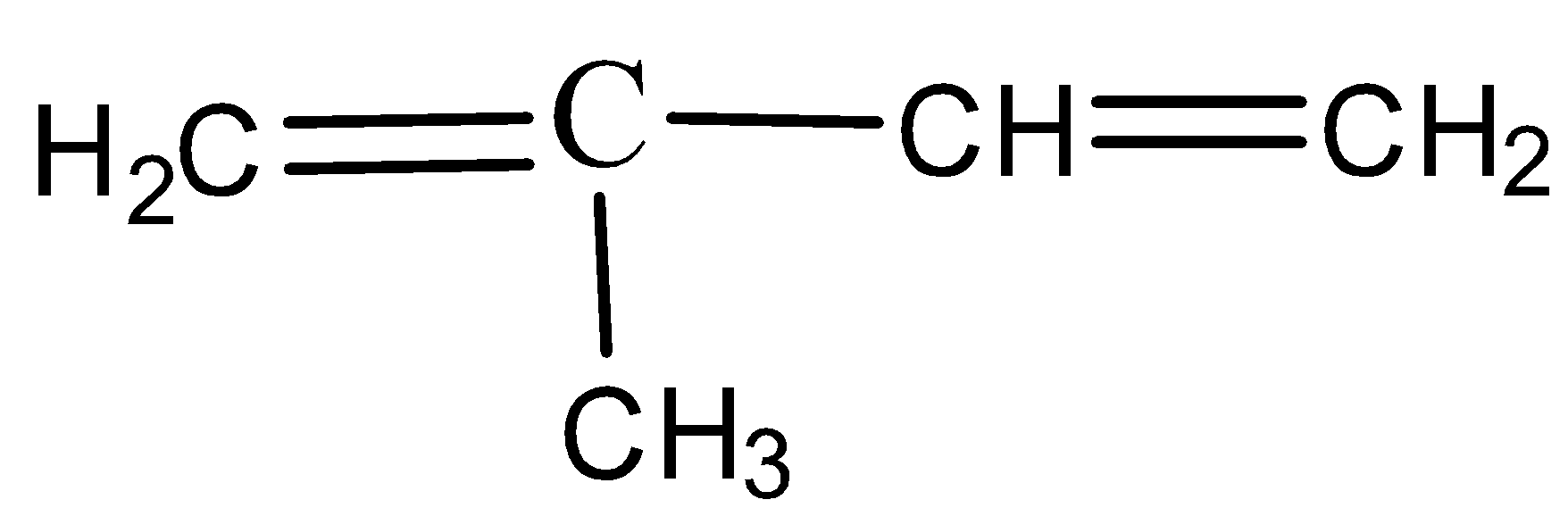
The polymerization process takes place in the presence of Ziegler-Natta catalyst and the reaction is as shown below:
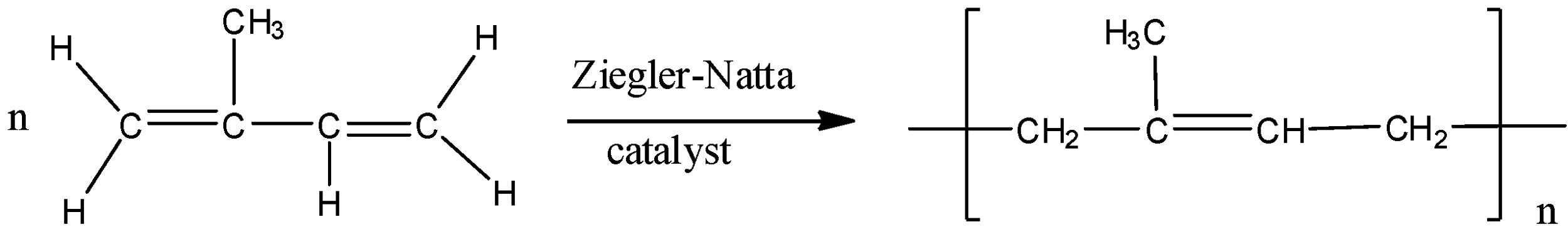
So the correct answer is “A”:
Note: The isoprene obtained naturally is a volatile liquid and is colourless and this unsaturated hydrocarbon is obtained even while processing petroleum or coal tar. Naturally it is obtained by trees like eucalyptus, oak etc.
