Question
Question: The monomer (\(G = Me\) or \(Cl\)) when treated with Zieglar-Natta catalyst undergo polymerisation a...
The monomer (G=Me or Cl) when treated with Zieglar-Natta catalyst undergo polymerisation as shown in the figure. Which of the following statements is not true considering the process given ?
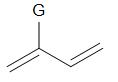
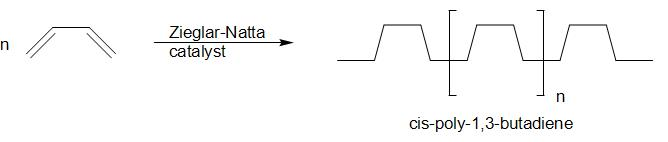
A.The general class of polymer formed is known as homopolymer
B.The polymer obtained is stereoregular
C.Buna-N can be prepared using above process
D.Synthetic rubber can be formed by above process using 1,3-butadiene
Solution
Ziegler-Natta catalyst is Triethyl Aluminium and titanium tetrachloride. Ziegler-Natta catalyst is used to polymerize terminal alkenes like ethylene and alkenes with the vinyl double bond. The polymer formed consists of a single species of monomer.
Complete step by step solution:
The polymer is formed by a single monomer species. Thus, this class of polymer is known as homopolymer.
Also, we can see that cis-poly-1,3-butadiene is stereoregular.
Since the above polymerisation reaction uses only one monomer, thus, Buna-N cannot be prepared using the given process. Buna-N is made up of the monomers 1,3-butadiene and acrylonitrile.
Synthetic rubber is derived from the copolymerization of styrene and 1,3-butadiene.
Thus, the correct options are A, B and D.
Note: The structure of active centres in the Ziegler–Natta catalysts is well established for metallocene catalysts. An idealized metallocene complex represents a typical pre catalytic. It is unreactive toward alkenes. A polymer molecule grows by numerous insertion reactions of carbon-carbon double bonds of terminal alkene molecules into the metal carbon bond in the ion.
Thousands of alkene insertion reactions occur at each active centre and results in the formation of long polymer chains attached to the centre. Polymerization reactions of alkene with solid titanium catalysts occur at special titanium centres located on the exterior of the catalyst crystals. Some titanium atoms in these crystallites react with organoaluminum cocatalysts with the formation of Ti–C bonds. The polymerization reaction of alkenes occurs almost similarly to the reactions of metallocene catalysts. Two chain termination reactions occur pretty rarely in the Ziegler–Natta catalysis and the polymers formed have a very high molecular weight to be of commercial use.
