Question
Question: The molecules having the same hybridization, shape and number of lone pairs of electrons are: A. \...
The molecules having the same hybridization, shape and number of lone pairs of electrons are:
A. SeF4, XeO2F2
B. SF4, XeF2
C. XeOF4, TeF4
D. SeCl4, XeF4
Solution
We have to determine the hybridisation and shape of the molecules. The mixing of two atomic orbitals with the same energy levels to form a new degenerate orbital is known as hybridisation. The arrangement of the electrons around the central atom is known as the shape of the molecule. The lone pair of electrons can be determined from the Lewis structures.
Complete step by step solution:
1. Determine hybridisation, shape and number of lone pairs of SeF4:
- The valence electrons of selenium are six and fluorine are seven. Thus, valence electrons of SeF4
⇒(1×Valence electrons of Se)+(4×Valence electrons of F)
⇒(1×6)+(4×7)
- Valence electrons of SeF4 =34
- The four fluorine atoms form four bonds with the central selenium atom. Thus, eight electrons are involved in bonding. Thus, the remaining electrons are,
⇒ Remaining electrons =34−8=26
- Place the remaining 26 electrons around the fluorine and selenium atoms such that all the atoms complete their octets.
Thus, the structure of SeF4 is as follows:
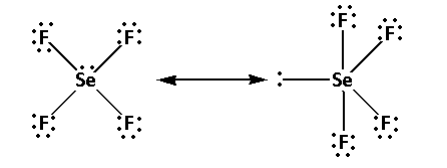
- From the structure of SeF4, we can conclude that there are four bond pairs and one lone pair.
- Thus, the hybridisation of SeF4 is sp3d and the shape is trigonal bipyramidal and the number of lone pairs is one.
2. Determine hybridisation, shape and number of lone pairs of XeO2F2:
The valence electrons of xenon are eight, oxygen are six and fluorine are seven. Thus,
Valence electrons of XeO2F2
⇒(1×Valence electrons of Xe)+(2×Valence electrons of O)+(2×Valence electrons of F)
⇒(1×8)+(2×6)+(2×7)
Valence electrons of XeO2F2 =34
- The two fluorine and two oxygen atoms form four bonds with the central xenon atom. Thus, eight electrons are involved in bonding. Thus, the remaining electrons are,
⇒ Remaining electrons =34−8=26
- Place the remaining 26 electrons around the fluorine, oxygen and xenon atoms such that all the atoms complete their octets.
Thus, the structure of XeO2F2 is as follows:
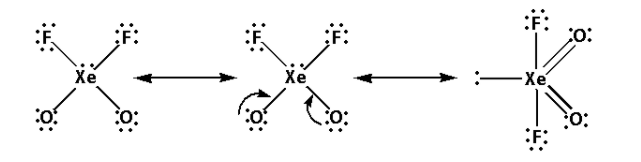
- From the structure of XeO2F2, we can conclude that there are four bond pairs and one lone pair.
- Thus, the hybridisation of XeO2F2 is sp3d and the shape is trigonal bipyramidal and the number of lone pairs is one.
- Thus, the hybridization, shape and number of lone pairs of electrons of SeF4, XeO2F2 are same.
Thus, option (A) is correct.
3. Determine hybridisation, shape and number of lone pairs of SF4:
- The valence electrons of sulphur are six and fluorine are seven. Thus,
Valence electrons of SF4
⇒(1×Valence electrons of S)+(4×Valence electrons of F)
⇒(1×6)+(4×7)
Valence electrons of SF4 =34
- The four fluorine atoms form four bonds with the central selenium atom. Thus, eight electrons are involved in bonding. Thus, the remaining electrons are,
⇒ Remaining electrons =34−8=26
- Place the remaining 26 electrons around the fluorine and selenium atoms such that all the atoms complete their octets.
Thus, the structure of SF4 is as follows:
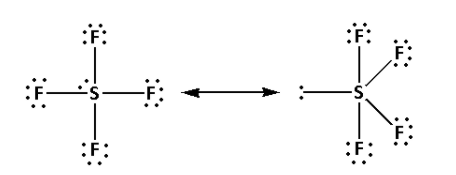
- From the structure of SF4, we can conclude that there are four bond pairs and one lone pair.
- Thus, the hybridisation of SF4 is sp3d and the shape is trigonal bipyramidal and the number of lone pairs is one.
4. Determine hybridisation, shape and number of lone pairs of XeF2:
The valence electrons of xenon are eight and fluorine are seven. Thus,
Valence electrons of XeF2
⇒(1×Valence electrons of Xe)+(2×Valence electrons of F)
⇒(1×8)+(2×7)
- Valence electrons of XeF2 =22
- The two fluorine atoms form two bonds with the central xenon atom. Thus, four electrons are involved in bonding. Thus, the remaining electrons are,
⇒ Remaining electrons =22−4=18
- Place the remaining 18 electrons around the fluorine and xenon atoms such that all the atoms complete their octets.
Thus, the structure of XeF2 is as follows:
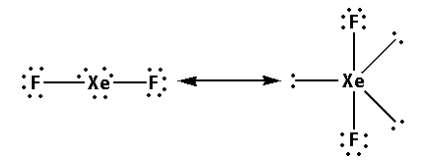
- From the structure of XeF2, we can conclude that there are two bond pairs and three lone pairs.
- Thus, the hybridisation of XeF2 is sp3d and the shape is trigonal bipyramidal and the number of lone pairs is three.
- Thus, the hybridization, shape and number of lone pairs of electrons of SeF4, XeF2 are not same.
Thus, option (B) is not correct.
5. Determine hybridisation, shape and number of lone pairs of XeOF4:
- The valence electrons of xenon are eight, oxygen are six and fluorine are seven. Thus,
Valence electrons of XeOF4
⇒(1×Valence electrons of Xe)+(1×Valence electrons of O)+(4×Valence electrons of F)
⇒(1×8)+(1×6)+(4×7)
Valence electrons of XeOF4 =42
- The four fluorine atoms and one oxygen atom form five bonds with the central xenon atom. Thus, ten electrons are involved in bonding. Thus, the remaining electrons are,
⇒ Remaining electrons =42−10=32
Place the remaining 32 electrons around the fluorine, oxygen and xenon atoms such that all the atoms complete their octets.
Thus, the structure of XeOF4 is as follows:
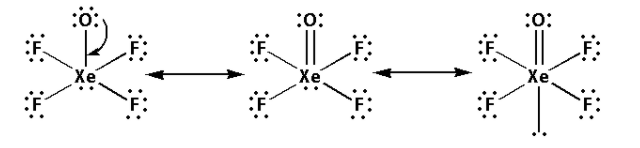
- From the structure of XeOF4, we can conclude that there are five bond pairs and one lone pair.
- Thus, the hybridisation of XeOF4 is sp3d2 and the shape is square pyramidal and the number of lone pairs is one.
6. Determine hybridisation, shape and number of lone pairs of TeF4:
- The valence electrons of tellurium are six and fluorine are seven. Thus,
Valence electrons of TeF4
⇒(1×Valence electrons of Te)+(4×Valence electrons of F)
⇒(1×6)+(4×7)
- Valence electrons of TeF4 =34
The four fluorine atoms form four bonds with the central tellurium atom. Thus, eight electrons are involved in bonding. Thus, the remaining electrons are,
⇒ Remaining electrons =34−8=26
Place the remaining 26 electrons around the fluorine and tellurium atoms such that all the atoms complete their octets.
Thus, the structure of TeF4 is as follows:
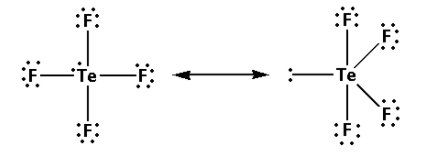
From the structure of TeF4, we can conclude that there are four bond pairs and one lone pair.
Thus, the hybridisation of TeF4 is sp3d and the shape is trigonal bipyramidal and the number of lone pairs is one.
Thus, the hybridization, shape and number of lone pairs of electrons of XeOF4, TeF4 are not same.
Thus, option (C) is not correct.
7. Determine hybridisation, shape and number of lone pairs of SeCl4:
- The valence electrons of selenium are six and chlorine are seven. Thus,
- Valence electrons of SeCl4
⇒(1×Valence electrons of Se)+(4×Valence electrons of Cl)
⇒(1×6)+(4×7)
Valence electrons of SeCl4 =34
The four chlorine atoms form four bonds with the central selenium atom. Thus, eight electrons are involved in bonding. Thus, the remaining electrons are,
⇒ Remaining electrons =34−8=26
- Place the remaining 26 electrons around the chlorine and selenium atoms such that all the atoms complete their octets.
Thus, the structure of SeCl4 is as follows:
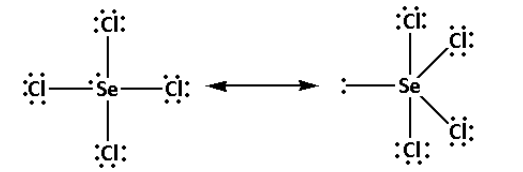
- From the structure of SeCl4, we can conclude that there are four bond pairs and one lone pair.
Thus, the hybridisation of SeCl4 is sp3d and the shape is trigonal bipyramidal and the number of lone pairs is one.
8. Determine hybridisation, shape and number of lone pairs of XeF4:
- The valence electrons of xenon are eight, oxygen are six and fluorine are seven. Thus,
Valence electrons of XeF4
⇒(1×Valence electrons of Xe)+(4×Valence electrons of F)
⇒(1×8)+(4×7)
Valence electrons of XeF4 =36
- The four fluorine atoms form four bonds with the central xenon atom. Thus, eight electrons are involved in bonding. Thus, the remaining electrons are,
⇒ Remaining electrons =36−8=28
Place the remaining 28 electrons around the fluorine and xenon atoms such that all the atoms complete their octets.
Thus, the structure of XeF4 is as follows:
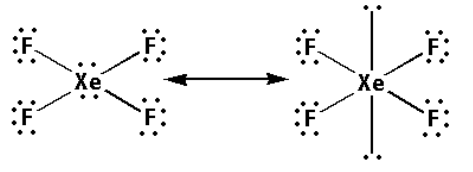
- From the structure of XeF4, we can conclude that there are five bond pairs and two lone pairs.
- Thus, the hybridisation of XeF4 is sp3d2 and the shape is octahedral and the number of lone pairs is one.
Thus, the hybridization, shape and number of lone pairs of electrons of SeCl4, XeF4 are not same.
Thus, option (D) is not correct.
Thus, the hybridization, shape and number of lone pairs of electrons of SeF4, XeO2F2 are same.
**Thus, the correct option is (A) SeF4, XeO2F2.
Note:**
To determine the shape of the molecule we must know the number of bond pairs and the number of lone pairs around the central atom. These can be determined by drawing the Lewis structures.
