Question
Question: The molecule with the highest dipole moment is: A.\[C{H_2}C{l_2}\] B.\[C{H_3}Cl\] C.\[CHC{l_...
The molecule with the highest dipole moment is:
A.CH2Cl2
B.CH3Cl
C.CHCl3
D.CCl4
Solution
To find the correct option, we must have basic knowledge of dipole moment, structures of all given molecules and the difference between bond dipole moment and net dipole moment. Here, the dipole moment arises due to electronegativity difference between chlorine atoms and hydrocarbons.
Complete step-by-step answer:
Dipole moment in any system arises due to the presence of separation of charges. They can arise in both, ionic as well as covalent bonds. Dipole moment occurs due to the electronegativity difference between two chemically bonded atoms and the measure of the polarity of a chemical bond between two atoms in a molecule is termed as the bond dipole moment of that molecule.
Let us look at the structure of each molecule and guess its dipole moment on that basis.
A. CH2Cl2
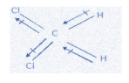
In this molecule, both chlorine groups contribute to the dipole moment but they are in different directions, disrupting the flow of electrons and the dipole moment as well. Here, one chlorine atom cancels half the effect of another chlorine atom.
B. CH3Cl
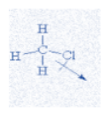
In this molecule, the dipole moment between C and H bond will be zero due to least electronegative difference. Therefore, only one chlorine group will contribute to the dipole moment of the molecule straight in one direction marking the highest dipole moment.
C. CHCl3
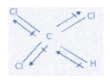
In this molecule, one of the chlorine atoms cancels the dipole moment of the opposite chlorine atom as both of them are lying equal and opposite to each other. So, the net dipole moment is just because of one chlorine atom.
D. CCl4
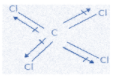
We already know that symmetrical molecules have zero dipole moment and this is a symmetrical molecule and nonpolar too. Therefore, its dipole moment will be zero as the chlorine atoms lie equal to opposite to each other, thereby cancelling each other’s effect.
This clears that amongst all the four molecules provided, CH3Cl will have the highest dipole moment.
Hence the correct option is (B).
Note: All these molecules have tetrahedral geometry due to sp3 hybridisation of carbon atoms and absence of unsaturated bonds. If we consider the case of a single bond in a polyatomic molecule, we call its dipole moment as the bond dipole moment and this dipole moment is different from the net dipole moment of the whole molecule.
