Question
Question: The molecule which has zero dipole moment is: (A)- \(C{{H}_{2}}C{{l}_{2}}\) (B)- \(B{{F}_{3}}\) ...
The molecule which has zero dipole moment is:
(A)- CH2Cl2
(B)- BF3
(C)- NF3
(D)- ClO2
Solution
The overall dipole moment is calculated by taking the vector sum of all the bonds present in the molecule. Also, the lone pairs contribute to the dipole moment of the molecule.
Complete step by step answer:
The dipole moment is present in the bond formed between two atoms which are partially charged due to their difference in electronegativity. It measures the polarity strength through the charges and the distance between them. Thus, a vector quantity as it defines the direction of partial flow of the electrons in the bond towards the more electronegative charged species or atom present in the molecule.
Its unit is coulomb meter or Debye, where the charge of the species is measured in coulombs and distance between them is measured in meters. When the molecule has more than two atoms and has more than one bond. Then, the vector sum of the individual dipole moments of the bonds are taken to measure the overall dipole moment in the molecule.
So, in the molecules given,
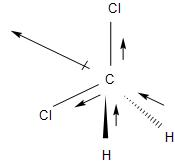
-The dichloromethane, CH2Cl2 has two C - H bonds with dipole moment directed towards the carbon atom and two C - F bonds dipole directed away from the carbon atom. Thus, the dipole moments are reinforcing each other and the overall dipole moment shown by the red arrow is not zero.
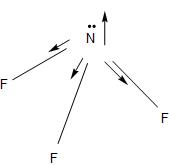
-The NF3 molecules having a pyramidal structure, has a lone pair and an unsymmetrical structure which gives it an overall positive dipole moment.
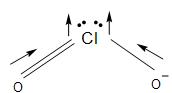
Also, in ClO2 molecule the two C=O bonds are in opposite directions, but the dipole is not cancelled due to the lone pair on the chlorine atom.
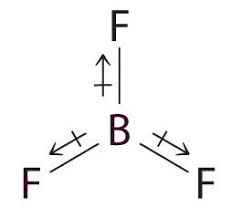
- But in case of BF3, the molecule having trigonal planar structure becomes symmetrical and gives zero dipole moment.
So, the correct answer is “Option B”.
Note: With the increase in the electronegativity difference between atoms and as their size increases the distance between their nuclei increases. Thus, increasing the dipole moment.
