Question
Question: The molecule which does not exhibit strong hydrogen bonding is: A. Methyl amine B. Diethyl ether...
The molecule which does not exhibit strong hydrogen bonding is:
A. Methyl amine
B. Diethyl ether
C. Acetic acid
D. Glucose
Solution
Hint: “Hydrogen bonding is a special type of dipole-dipole attraction or bond between molecules”. It is not a covalent bond. Only those compounds in which Hydrogen atom is attached directly with a highly electronegative atoms i.e. N, O or F are able to show the property called hydrogen bonding.
Complete step by step answer:
- Hydrogen bonding is a weak attractive force formed between different molecules.
- Coming to given options, Option A, Methyl amine.
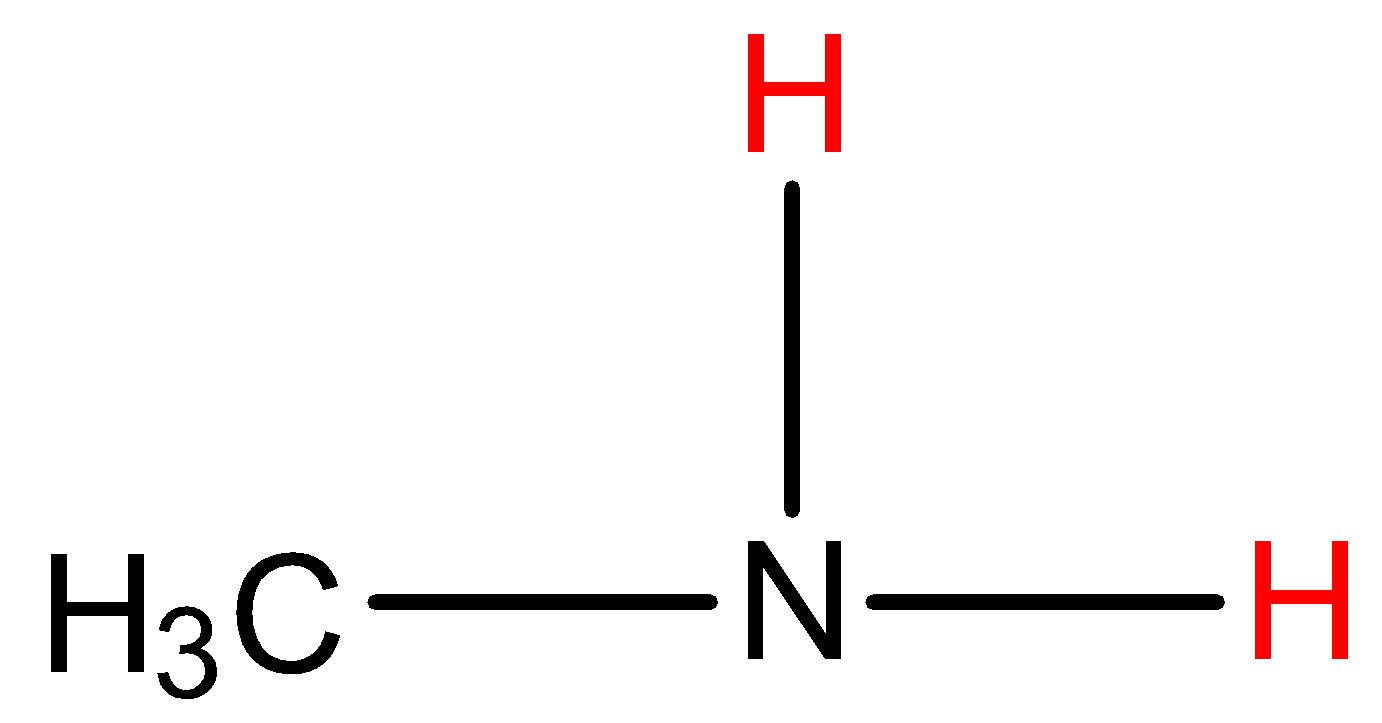
- In the structure the red marked hydrogens are directly attached with nitrogen which has high electronegativity. So, methylamine shows hydrogen bonding with other methyl amine molecules.
- Coming to option B, diethyl ether.
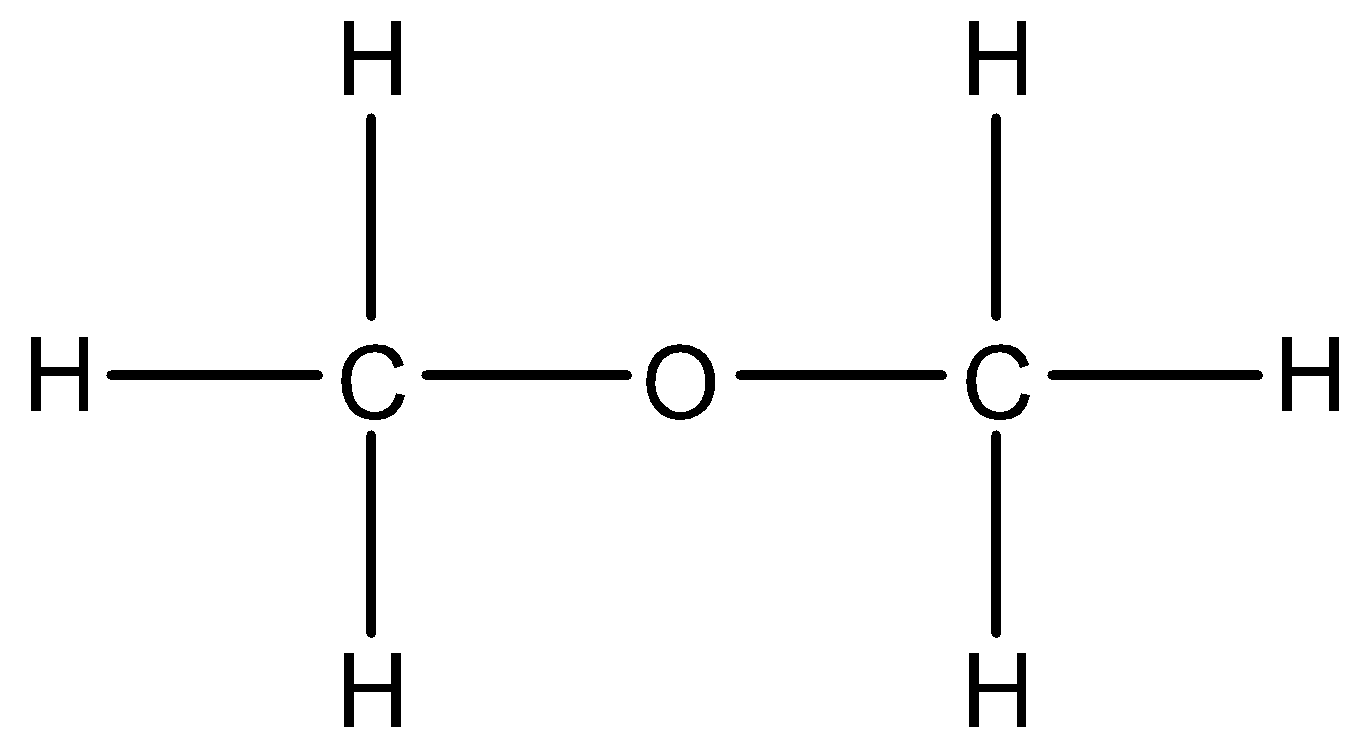
- In diethyl ether the hydrogen is not directly attached to high electronegativity atoms. So, diethyl ether does not show hydrogen bonding.
- Coming to option C, acetic acid.
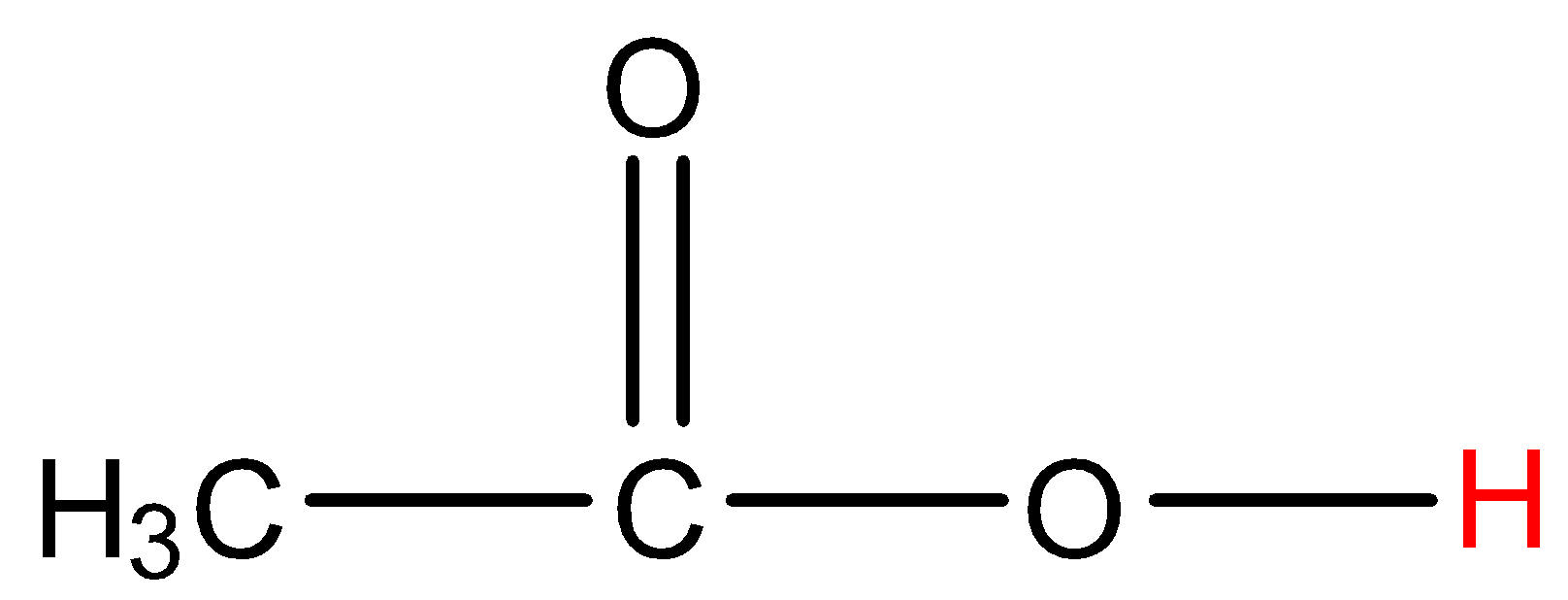
- The hydrogen which is marked with red color is directly attached to a highly electronegative atom. So, acetic acid shows hydrogen bonding.
- Coming to option D, Glucose.
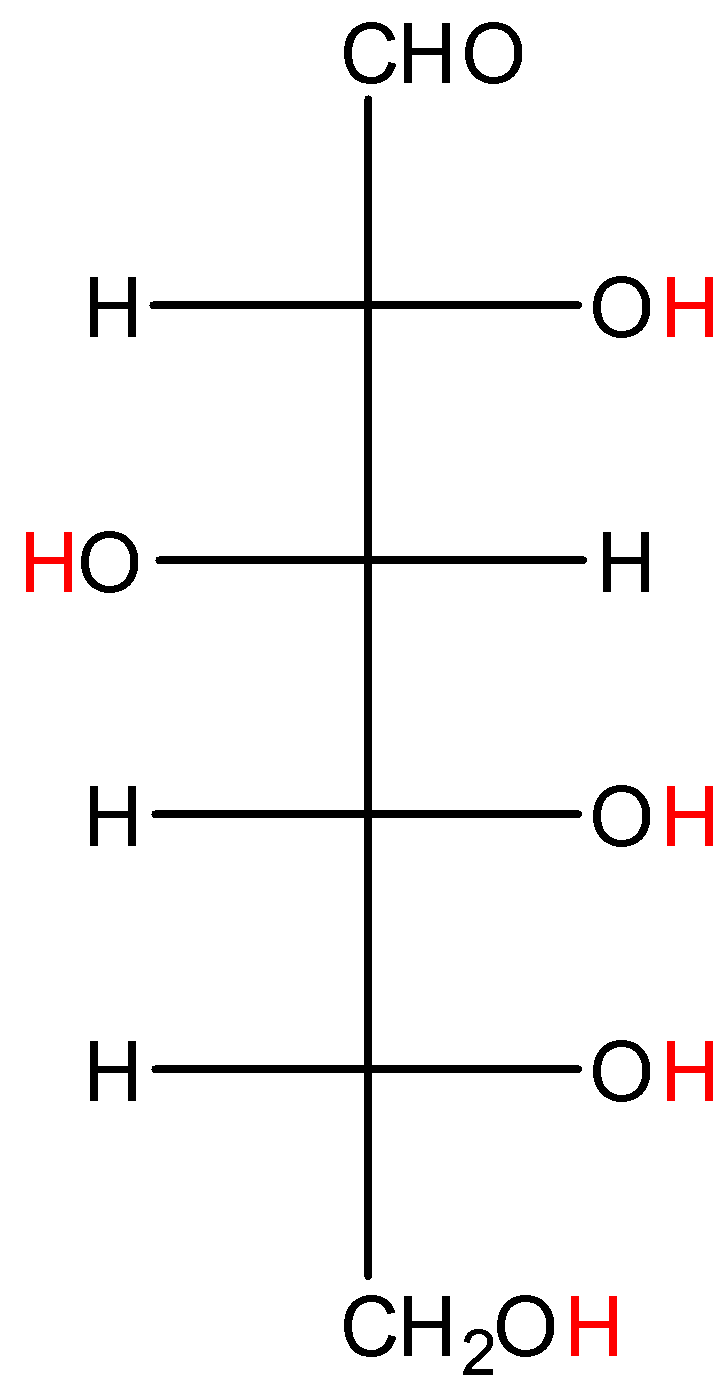
- In glucose also the hydrogens marked with red color are directly attached to high electronegativity atoms. So, glucose shows hydrogen bonding.
So, the correct option is B, diethyl ether won’t show the hydrogen bonding.
Note: Don’t be confused with the words, hydrogen bond and covalent bond. Both are different.
Covalent bond: Covalent bond is going to form between two atoms having less electronegativity difference.
Hydrogen Bond: Hydrogens which are attached to high electronegative atoms like N, O, F shows the hydrogen bonding with the other molecules.
