Question
Question: The molecule which does not exhibit dipole moment is: (a)- \(N{{H}_{3}}\) (b)- \(CHC{{l}_{3}}\)...
The molecule which does not exhibit dipole moment is:
(a)- NH3
(b)- CHCl3
(c)- H2O
(d)- CCl4
Solution
Draw the structure of all the compounds in the options and check their symmetry. To draw the dipole moment in the molecule, find the more electronegative atom joining the bond and draw the arrow towards the electronegative atom. If the molecule has a symmetry then the dipole will be zero.
Complete answer:
The dipole moment is calculated for the polar molecule, as the polar molecule has two different ends, i.e., a positive end and a negative end. The negative end represents the more electronegative atom and the positive end represents the less electronegative atom.
To draw the dipole moment in the molecule, find the more electronegative atom joining the bond and draw the arrow towards the electronegative atom. If the molecule has a symmetry then the dipole will be zero.
Let us draw all the structures of the compounds given in the question.
(a)- NH3
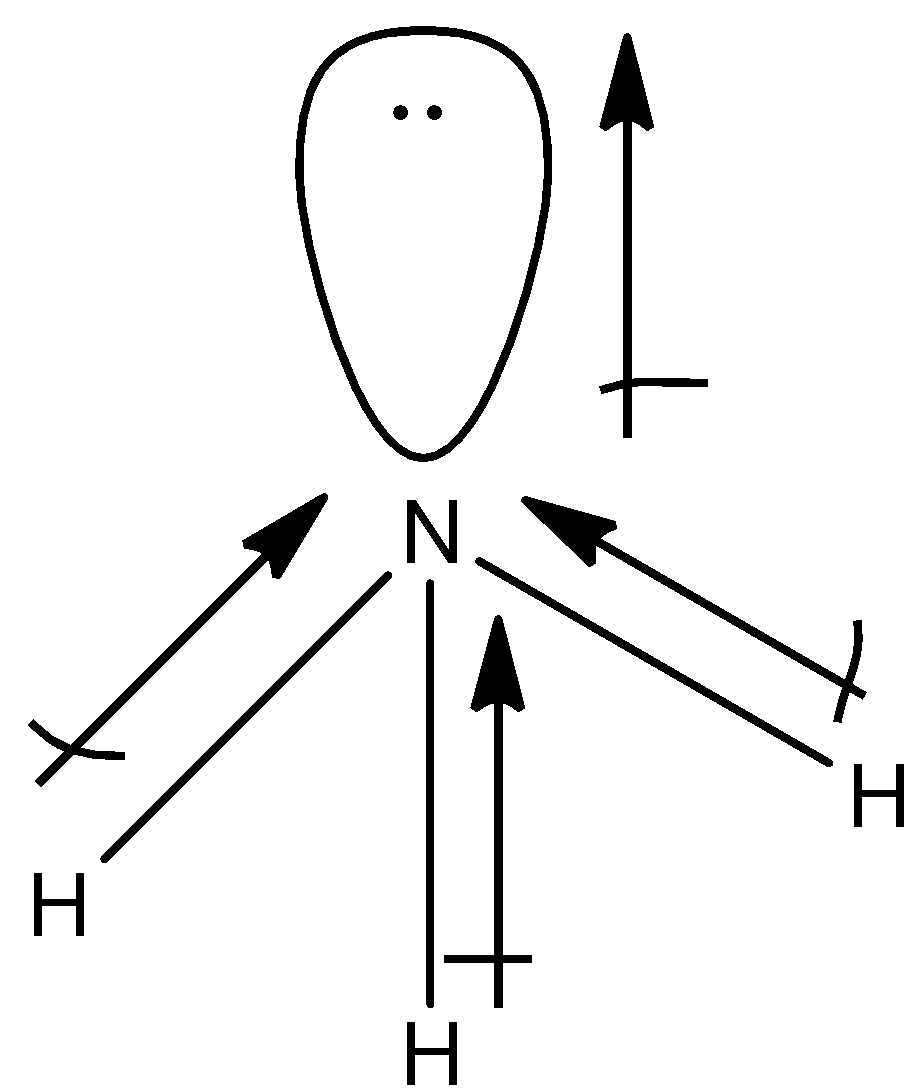
This is not a symmetrical molecule and the dipole moment of NH3 is non-zero. So, the dipole moment is 1.47D.
(b)- CHCl3
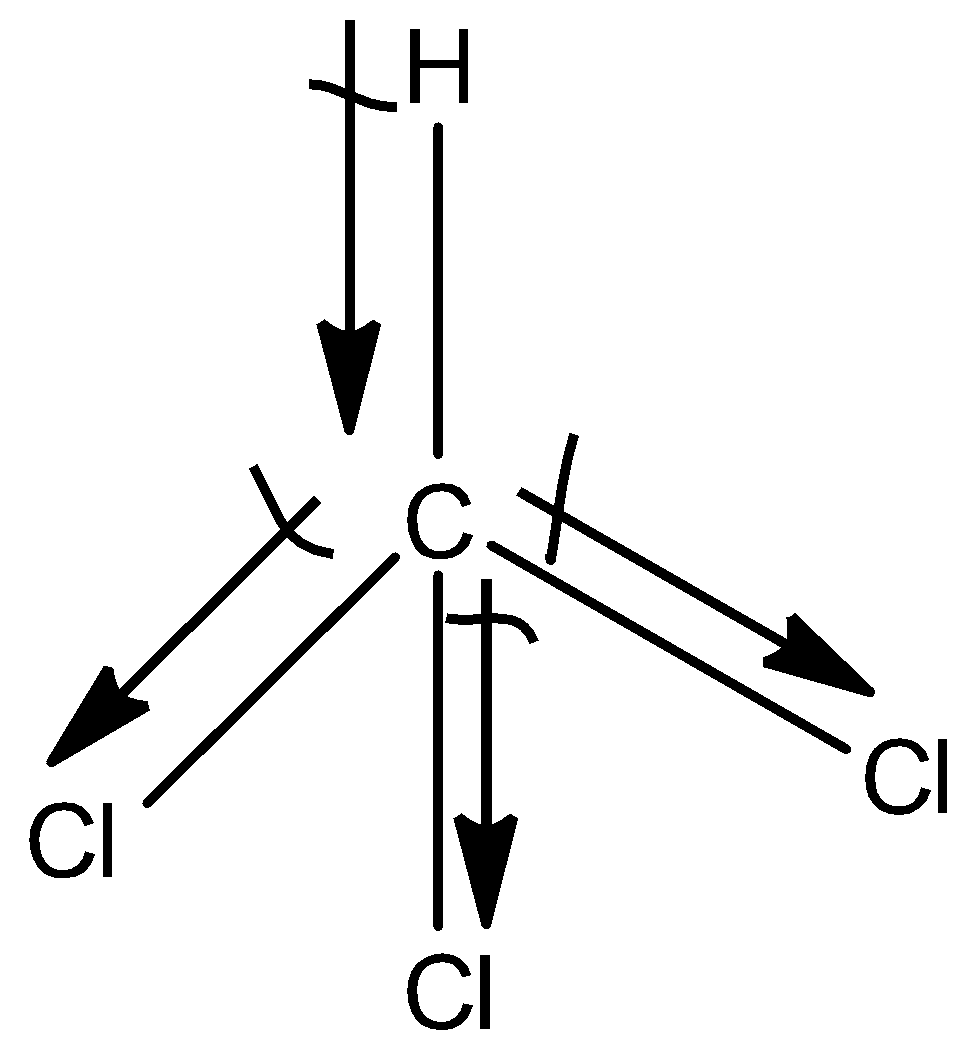
This is a symmetrical molecule but the atoms attached to the carbon atoms are different so, the dipole moment of CHCl3 is also non-zero. So, the dipole moment is 1.04D.
(c)- H2O
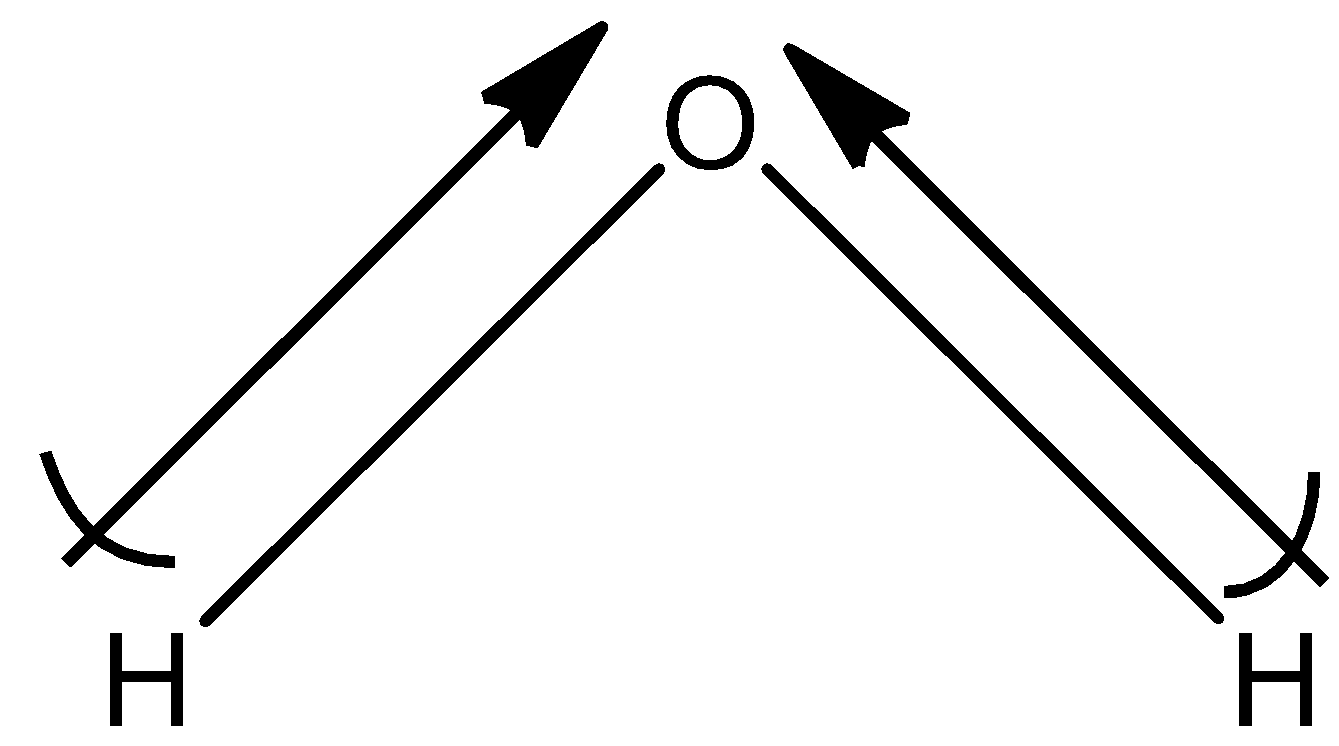
This is not a symmetrical molecule and the dipole moment of H2O is non-zero. So, the dipole moment is 1.84D.
(d)- CCl4
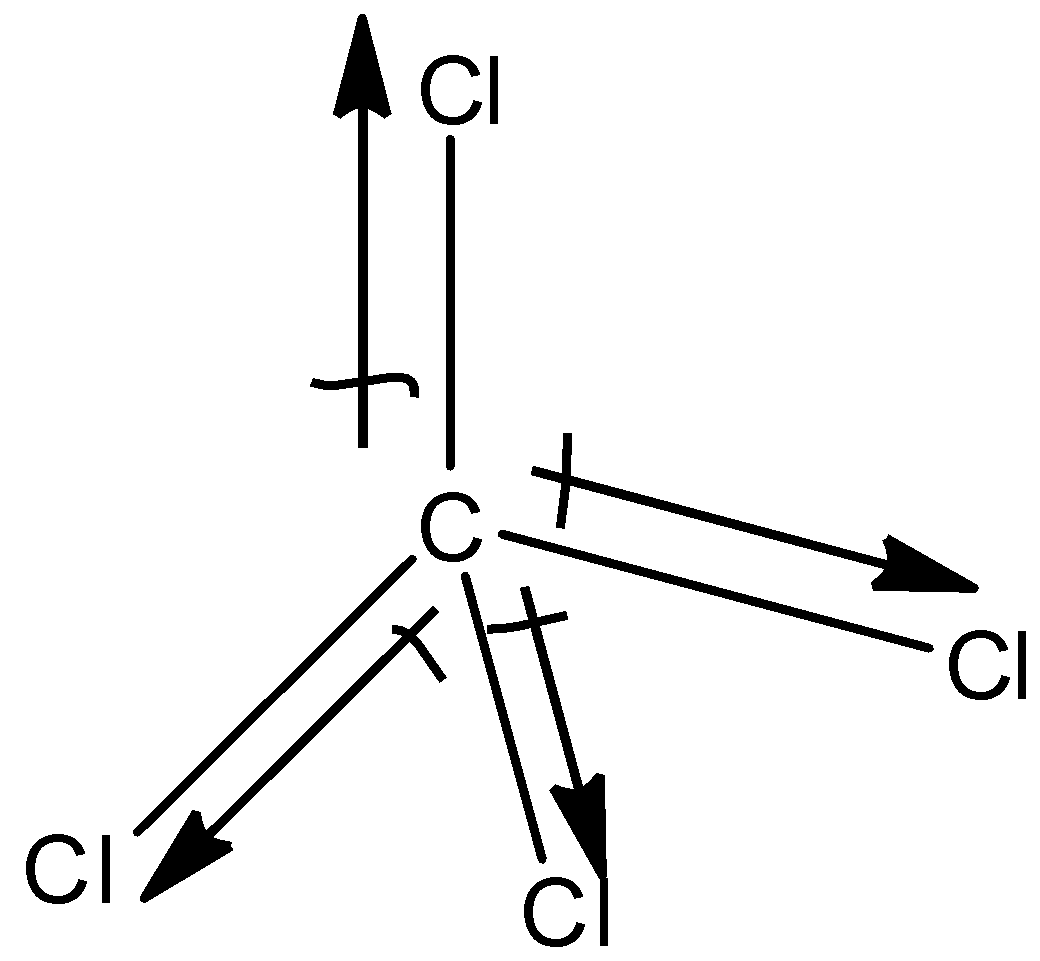
This is a symmetric molecule and all the atoms attached to the carbon atom are the same, so the dipole moment of CCl4 is zero and it does not exhibit dipole moment.
Therefore, the correct answer is option (d)- CCl4.
Note:
The dipole moment is defined mathematically as the product of the magnitude of a negative or positive charge (q) and the distance (d) between the centers of positive and negative charges mathematically.
