Question
Question: The molecule that have dipole moment: A. 2, 2-dimethyl propane B. Cis-3-hexene C. 2,2,3,3-tetr...
The molecule that have dipole moment:
A. 2, 2-dimethyl propane
B. Cis-3-hexene
C. 2,2,3,3-tetramethyl butane
D. None of these
Solution
Dipole moments occur in any system where there is a separation of charge. They can occur in ionic bonds as well as covalent bonds. It arises due to the electronegativity difference between the bonded atoms. The dipole moment in a bond is the measure of the polarity of the chemical bonds between two atoms in a molecule. The concept involves the separation of the negative and positive charges in a bond. It has a magnitude and direction due to which is a vector quantity. For example, showing the dipole moment of HCl (hydrochloric acid) in the diagram below:
δ+H−Clδ−
Complete step by step answer:
The first option is 2, 2-dimethyl propane. The structure of 2, 2-dimethyl propane is
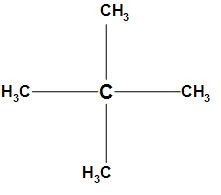
In the structure of 2, 2-dimethyl propane the sp3 hybridized carbon which is electropositive will attract the electrons from all the methyl groups towards itself and due to which a dipole is created. Hence, the opposite dipole moments will cancel each other and the resultant dipole moment will be zero. Thus, 2, 2-dimethyl propane has no dipole moment.
Second option is Cis-3-hexene. The structure of Cis-3-hexene is
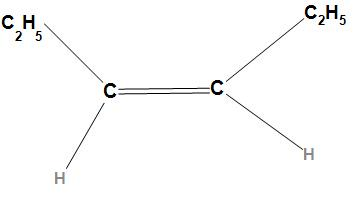
In the structure of Cis-3-hexene, the sp2 hybridized carbon which is more electropositive than the sp3 hybridized carbon will attract the electrons towards itself due to which a dipole will be created. Hence there is no opposite dipole moment, it will not cancel each other and hence the resultant dipole moment will not be zero. Thus, Cis-3-hexene has a dipole moment.
The third option is 2, 2, 3, 3-tetramethyl butane. The structure of 2, 2, 3, 3-tetramethyl butane is:
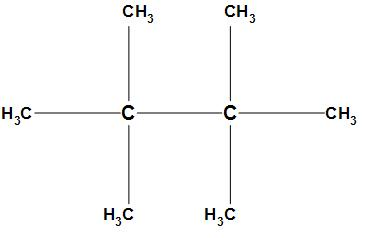
In the structure of 2, 2, 3, 3-tetramethyl butane the sp3 hybridized carbon which is electropositive will attract the electrons from all the methyl groups towards itself due to which a dipole will be created. The opposite dipoles will cancel each other and the resultant dipole moment will be zero. Thus, 2, 2, 3, 3-tetramethyl butane has no dipole moment.
After discussing we can conclude that Cis-3-hexene has a dipole moment.
So, the correct answer is Option B .
Note: Cis-3-hexene is symmetrical disubstituted alkene. It is prepared by the hydroboration of 3-hexyne which on protonolysis gives the final Cis-3-hexene. The sp2 hybridized carbon in Cis-3-hexene is electropositive.
