Question
Question: The molecule of magnesium chloride is linear, whereas that of stannous chloride is angular. If tru...
The molecule of magnesium chloride is linear, whereas that of stannous chloride is angular.
If true enter 1, else enter 0.
Solution
First check the number of valence electrons in Mg and Sn atoms of both compounds and their bonding with the 2 Cl atoms. Also check for the presence of any lone pairs and try to deduce the structure. Calculate the hybridisation using the formula given below and see the structure related to it.
H=1/2[V+M−C+A]
Complete step by step answer:
-First of all we will start by calculating the hybridisation of magnesium chloride (MgCl2) and checking its shape. For finding out the hybridisation we will use the following formula:
H=1/2[V+M−C+A] (1)
Where, V=number of valence electrons;
M=monovalent atoms;
C=positive charge;
A=negative charge.
Hybridisation of MgCl2 will be: H=21[2+2−0+0]
= 24 = 2
Since the hybridisation number for it is 2, its hybridisation will be sp and its shape will be linear.
This can also be explained as: Mg has 2 electron clouds formed from its bonding with the 2 Cl atoms. The chlorine atom being highly electronegative attracts an electron from the magnesium atom and forms a bond. Also the number of valence electrons in Mg is only 2 and both are involved in bonding with 2 Cl atoms, so there are no lone pairs. Its structure is one-dimensional and the atoms are connected in straight lines. Also the bond angle here is 180∘ . Its structure will be:

So, we can finally say that the structure of magnesium chloride (MgCl2) is linear.
-We will now talk about the structure of stannous chloride (SnCl2).
First let us calculate its hybridisation using equation (1).
H=21[4+2−0+0]
= 3
The hybridisation number is 3 and so hybridisation will be sp2. Out of the 4 valence electrons in Sn, 2 are bonded with the 2 Cl atoms and there is a lone pair of electrons left. So, it forms a tetrahedral structure.
This can also be explained as: SnCl2 is an angular covalent molecule due to the repulsion between the lone pair of electrons on Sn atom and the two bonds with the 2 chlorine atoms.
Its structure will be:
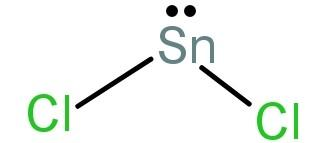
-As we have proved above the given statement: “The molecule of magnesium chloride is linear, whereas that of stannous chloride is angular” is true.
Note: Hybridisation does not only depend on the number of bonds formed in the given compound. The presence or absence of any lone pairs also plays a major role in it because the presence of lone pair causes lone pair-bond pair repulsion leading to the angularity of the structures.
