Question
Question: The molecule \(B{F_3}\) and \(N{F_3}\) both are covalent compounds but \(B{F_3}\) is non-polar and \...
The molecule BF3 and NF3 both are covalent compounds but BF3 is non-polar and NF3 is polar, the reason is that:
(A) Boron is a metal and nitrogen is a gas in uncombined state
(B) BF3 bond have no dipole moment whereas NF3 bond have dipole moment
(C) Atomic size of boron is smaller than that of nitrogen
(D) BF3 is symmetrical molecule whereas NF3 is unsymmetrical
Solution
Both boron trifluoride, BF3 and nitrogen trifluoride, NF3are formed by covalent bonding that means the bonds in these compounds are formed by sharing of one or two pairs of valence, electrons. Covalent bonding is very common in compounds formed by combining two non-metals.
Complete step by step solution:
The covalent compounds can be polar or non-polar, depending upon the polarity of bonds involved in these compounds.
In polar covalent compounds, the bonding present is of polar covalent bonding. The bond is formed in two different atoms which have different electronegativity that means there is an unequal sharing of electrons during bond formation between two atoms. The unequal sharing of electrons leads to the development of fractional positive and negative charge on the atoms in a bond and the bond is said to become polar as in NF3.
In non-polar compounds, the bonding is a non-polar type that means the electrons are shared between bonded atoms equally. Usually, the electronegativity difference between two bonded atoms in a non-polar bond is less than 0.5. The formation of non-polar covalent bonds may take place if bonded atoms in a polar covalent bond cancel the charges on them by arranging accordingly.
Polarity and non-polarity of molecules depend upon their dipole a moment. Let us first understand the dipole moment of a molecule.
During covalent bond formation, when there is an unequal sharing of electrons between atoms, the separation of charges takes place between atoms resulting in a dipole moment. The dipole moment is denoted as μ and measured in the Debye unit. The shape of a molecule with dipole moment and the polarity of its bonds are the determining factors for deciding the overall polarity of that molecule. The molecule may contain a polar bond but, the molecule may not be polar.
Let us now understand the structure and bonding in both BF3 and NF3.
The molecule of BF3 contains 1 boron atom and 3 fluorine atoms containing 3 lone pairs of electrons on each fluorine atom. The structure of BF3 is given below.
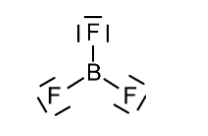
Also according to VSEPR, the BF3 molecule has trigonal planar geometry and each F−B−F is of 120°. The BF3 molecule is highly symmetrical due to its geometry. Also, the dipole moment of a molecule is a vector quantity that means it requires both magnitude and direction. The three bonds in BF3 are polar and they have dipole moments as shown below.
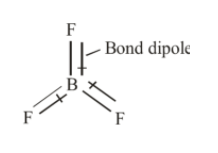
The bond dipole moments in BF3 are cancelled due to its symmetrical geometry resulting in a net dipole moment of BF3 equal to zero. Hence, BF3 is non-polar.
The molecule of NF3 contains 1 nitrogen atom and 3 fluorine atoms containing 1 lone pair of electrons on nitrogen atom. The structure of NF3 is given below.
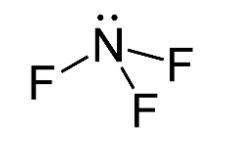
In the molecule NF3, three fluorine atoms one electron each to form covalent bonds with nitrogen atoms and nitrogen atom is left with a lone pair of electrons. The repulsion between lone pair of electrons on nitrogen and bond pairs of electrons NF3 molecule leads to bent shape of molecule and the resulting geometry is trigonal pyramidal. Hence, the three dipoles on N−F bond originate towards downward direction and one dipole is in upward direction because of lone pair of electrons. The unsymmetrical geometry of NF3 molecule causes a non-zero dipole moment in the molecule.
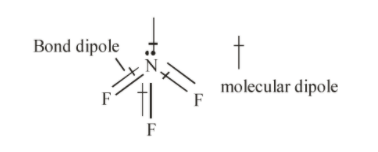
The molecule BF3 and NF3 both are covalent compounds but BF3 is non-polar and NF3 is polar, the reason is that BF3 is symmetrical molecule whereas NF3 is unsymmetrical.
Hence, the correct answer is option (D).
Note: The compound NF3is polar as the sharing of valence electrons between atoms in the compound does not take place equally. On the other hand, BF3is a polar compound as electrons are shared equally by atoms in a molecule.
The dipole moment is the product of magnitude of the charge and the distance between the centres of positive and negative charges.
Therefore, mathematically dipole moment can be expressed as,
DipoleMoment=charge×distanceseparation
μ=δ×d
Here, μis the dipole moment of a bond, δ represents the magnitude of partial charges δ+ and δ−. The letter d represents the distance between partial charges δ+ and δ−.
