Question
Question: The molecular formula of the first member of alkenynes and its name is given by the set: \(A.\;{C_...
The molecular formula of the first member of alkenynes and its name is given by the set:
A.C3H2, Alkene
B.C5H6, Pent-1-en-3-yne
C.C6H8, Hex-1-en-5-yne
D.C4H4, But-1-en-3-yne
Solution
Hint: Alkynes is a hydrocarbon, in which a triple bond binds two carbon atoms. The simplest alkyne would therefore have 2 carbon atoms within its molecule.
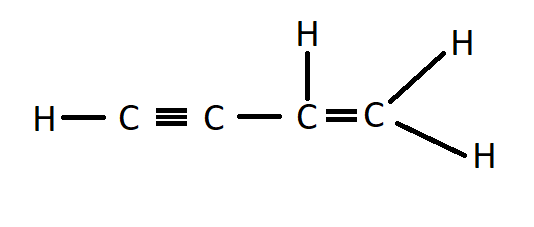
First member of Alkenynes
Complete answer:
The general formula for alkynes is CnH2n−2 where C is the Carbon atom and H is Hydrogen atom and n = 2,3,4,5.......
We will call the structure as meth, eth, prop, but, pent, hex, hept, oct, non, dec etc according to the number of carbon atoms present.
Usually we don't use meth as the simplest Alkyne has 2 carbon atoms and meth is used for 1 carbon atom, so we can use eth directly
For example, when we substitute the value of n = 2 in the formula CnH2n−2, we get C2H2
To nomenclature this compound we will apply 'yne' to the number of carbon atoms used as the primary suffix. And here we used 2 carbon atoms, the name of the compound will be :- Eth + yne = Ethyne
C4H4 will be the first member of the alkenyes group and no other compound can be smaller than this compound.
Hence, option D is the correct answer, which is C4H4, But-1-en-3-yne is the first member of alkenynes.
Note: While solving such types of problems we must remember the properties of Carbon atoms and the properties of alkenynes. In this alkenynes is given by CnH2n−2 and the smallest compound that can exist is C4H4, But-1-en-3-yne. Also, we must know how to do the naming of the compound.
