Question
Question: The molecular formula of dithionic acid is: A.\({H_2}{S_2}{O_4}\) B.\({H_2}{S_2}{O_6}\) C.\({H...
The molecular formula of dithionic acid is:
A.H2S2O4
B.H2S2O6
C.H2S2O5
D.H2S2O7
Solution
Dithionic acid is the oxoacid of sulphur. Oxoacids are those acids which are identified by the presence of oxygen atoms in an acid. In oxoacid oxygen is bonded to hydrogen atom. There are different types of oxoacids of sulphur that exist.
Complete answer:
The structure of dithionic acid is given below as follows:
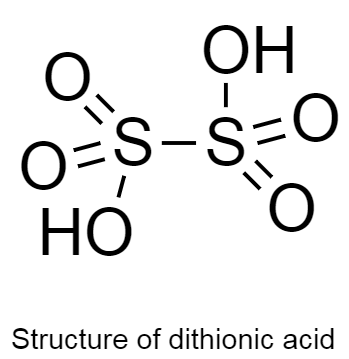
In the given structure there are two hydrogen atoms bonded to two oxygen atoms.
There are two hydrogen, six oxygen and two sulphur atoms in the structure.
From this we can say that the molecular formula of dithionic acid is H2S2O6 .
The oxidation state of sulphur in dithionic acid is +5 .
The oxoacids of sulphur are tetrahedral in structure when they are bonded to oxygen atoms.
These oxoacids contain at least one sulphur-oxygen double bond and one sulphur-hydroxide bond.
So, the correct answer is option b) H2S2O6
Additional information:
Some examples of oxoacids of sulphur are namely: sulphuric acidH2SO4 , Sulfurous acid H2SO4 , peroxodisulfuric acid H2S2O8 .
Thionic acids are unstable compared to other oxoacids of sulphur.
Oxyacids dissociate in water to form acid anion and hydrogen cations.
These oxyacids are only important for the salts that are obtained from them.
Otherwise they do not have any other application.
Sulphuric acid is the only oxoacid that is used widely in industries for numerous purposes.
Note: The electronegativity of the central metal atom and the total number of oxygen atoms are responsible for the acidic character in oxoacids.
The hydrogen that is bonded to oxygen in oxyacids is acidic in nature.
