Question
Question: The molecular formula of dithionic acid is: (A) \[{H_2}{S_2}{O_7}\] (B) \[{H_2}{S_2}{O_6}\] (C...
The molecular formula of dithionic acid is:
(A) H2S2O7
(B) H2S2O6
(C) H2S2O5
(D) H2SO5
Solution
In order to determine the molecular formula of dithionic acid, we must know about the structure of dithionic acid. Dithionic acid is said to be an oxo acid of Sulphur. It is diprotic in nature. It will act as mild oxidizing as well as mild reducing agent.
Complete step by step answer:
The free acid of Dithionic acid is not known. It is always present in solution form. Dithionic acids are diprotic in nature. The salts of Dithionic acid are known as the Dithionates. Dithionates are soluble in water. They are the mild reducing as well as the mild oxidizing agent. The Dithionate will be having a structure similar to Ethane.
The structure of Dithionic acid is given below:
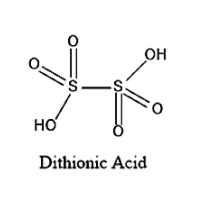
On seeing the above given structure, we can say that the Molecular formula of Dithionic acid is H2S2O6.
Discussing option, (A), the formula H2S2O7, suggests us pyrosulfuric acid.
Discussing option (C), the formula H2S2O5, suggests hyposulfurous acid.
Discussing option (D), the formula H2SO5 suggests Peroxymonosulfuric acid or Caro’s Acid.
Therefore, the correct answer is option (B) H2S2O6.
Additional information:
We can prepare Dithionates by the following methods:
Dithionite can be prepared by the oxidation of sulphite, i.e. the oxidation state changes from +4 to +5. It can also be prepared by oxidizing aqueous solution of Sulphur dioxide with manganese dioxide.
2MnO2+3SO2→MnS2O6+MnSO4
Barium dithionate solution on treatment with sulfuric acid will give Dithionic acid.
BaS2O6(aq)+H2SO4(aq)→H2S2O6(aq)+BaSO4(s)↓
Note: Sulphur has various oxoacids. They are:
- Sulphuric acid
- Sulphurous acid
- Permono Sulphuric acid
- Perdi Sulphuric acid
- Thiosulphuric acid
- Dithionic acid
- Pyrosulphuric acid
