Question
Question: The molecular formula of chlorine is \(C{l_4}\) A. True B. False...
The molecular formula of chlorine is Cl4
A. True
B. False
Solution
b>Hint:** Chlorine is a chemical element with the symbol Cl and atomic number 17. It is the second lightest element of the halogens, and it appears between fluorine and bromine in the periodic table. It is yellow-green gas at room temperature.
Complete step by step answer:
Chlorine is a green yellow gas with a very pungent odor that is twice as dense as air. It is basically a chemical element that belongs to the halogen group with the symbol Cl. It was discovered in 1770 and soon became useful as a commercial agent.
The molecular formula of this gas is Cl2 and it is a diatomic molecule which means that it has two atoms of the same element in the molecule. Its structure is as shown:
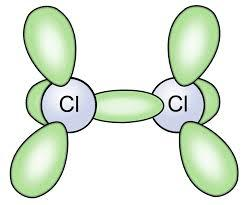
Moreover, chlorine reacts with organic compounds and ammonia to form chloro-organics or chloramines. These are the part of the group of chlorine compounds that have disinfectant properties and show up as part of chlorine.
It also acts as a reducing agent present in wastewater. These reactions are called chlorine demand. The reaction is as shown:
H2O+Cl2→HCl+HClO
Therefore, in the given question the molecular formula of chlorine is wrong.
Hence, it is false. Option B is correct.
Note: Chlorine gas was used by the Germans as a chemical weapon against the allied troops during the First World War. It is an intermediate water-soluble pulmonary irritant that causes acute damage in the upper and lower respiratory tract. Furthermore, it is also used to prevent the spread of various waterborne diseases.
