Question
Question: The molecular formula \({C_3}{H_9}N\) cannot represent : ( A ) \(1^\circ \) amine ( B ) \(2^\cir...
The molecular formula C3H9N cannot represent :
( A ) 1∘ amine
( B ) 2∘ amine
( C ) 3∘ amine
( D ) Quaternary salt
Solution
There are only 3−C atoms present in the molecular formula so only those structures are possible according to the formula which are having 3−C atom. Quaternary salt requires 4−C atoms .
Complete step-by-step answer: First let’s see the structural isomers of this molecular formula :
1. CH3−CH2−CH2−NH2 → Propan−1−amine ( 1∘ amine )
2. CH3−NH−CH2−CH3 → N−methylethanamine (2∘ amine )
3. 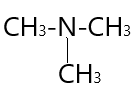 → N,N−Dimethylmethanamine( 3∘amine )
→ N,N−Dimethylmethanamine( 3∘amine )
4. 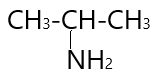 \to $$$Propan - 2 - amine\;$$ (1^\circ $ amine )
\to $$$Propan - 2 - amine\;$$ (1^\circ $ amine )
There are 4 structural isomers of the molecular formulaC3H9N according to the number of carbon atoms , number of hydrogen atoms and number of nitrogen atoms .
As this molecular formula has 3−Catom , 9−H atom , 1−N atom . As we can see that molecular formula has only 3−C atoms so only these 4 structures are possible: 1∘ amine , 2∘ amine , 3∘ amine .
While for Quaternary salt, we require 4−carbon atoms ,which according to the molecular formula is not possible.
In ( 1 ) structural isomer −NH2 group attaches to the one carbon atom and results in the 1∘ amine.
In ( 2 ) structural isomers −NH2 group attach to two carbon atoms and result in the formation of 2∘ amine .
In ( 3 ) structural isomer −NH2 group attached to three carbon atoms and result in the formation of 3∘amine .
In ( 4 ) structural isomer −NH2 group attached to one carbon atom and results in the formation of 1∘ amine.
Note: Structural isomers depend on the number of atoms present in the given molecular formula. So we have to count the number of atoms given in the molecular formula .
