Question
Question: The molar solubility of lead(II) azide, $Pb(N_3)_2$, in a buffer solution with pH = 1.00, is _____ $...
The molar solubility of lead(II) azide, Pb(N3)2, in a buffer solution with pH = 1.00, is _____ molL−1. Given that
Pb(N3)2(s)⇌Pb2+(aq)+2N3−(aq);Ksp=2.5×10−9
HN3(aq)+H2O(l)⇌H3O+(aq)+N3−(aq);Ka=2.0×10−5
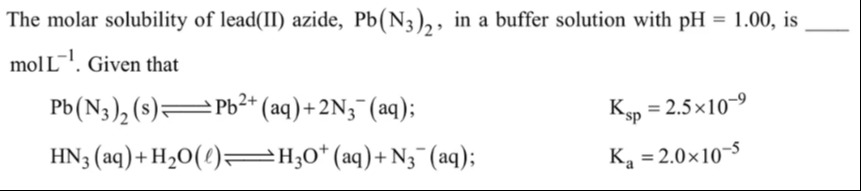
0.25
Solution
The molar solubility of lead(II) azide, Pb(N3)2, in a buffer solution with pH = 1.00 needs to be calculated.
The dissolution equilibrium of Pb(N3)2 is: Pb(N3)2(s)⇌Pb2+(aq)+2N3−(aq)
The solubility product constant is Ksp=[Pb2+][N3−]2=2.5×10−9.
Let S be the molar solubility of Pb(N3)2. Then, at equilibrium, [Pb2+]=S.
The total concentration of azide species originating from the dissolution of Pb(N3)2 is 2S. These azide species can exist as N3− or HN3 in the solution due to the acid-base equilibrium of the azide ion.
The azide ion (N3−) is the conjugate base of the weak acid hydrazoic acid (HN3). The equilibrium is: HN3(aq)+H2O(l)⇌H3O+(aq)+N3−(aq)
The acid dissociation constant is Ka=[HN3][H3O+][N3−]=2.0×10−5. Let [H+] represent [H3O+].
The pH of the buffer solution is given as 1.00, so [H+]=10−pH=10−1.00=0.10M.
From the Ka expression, we can relate the concentrations of N3− and HN3: [HN3]=Ka[H+][N3−]=2.0×10−50.10M[N3−]=2×10−510−1[N3−]=0.5×104[N3−]=5000[N3−].
The total concentration of azide species is the sum of the concentrations of N3− and HN3: 2S=[N3−]+[HN3]
Substitute the expression for [HN3] in terms of [N3−]: 2S=[N3−]+5000[N3−]=5001[N3−] So, [N3−]=50012S.
Now substitute the concentrations of Pb2+ and N3− into the Ksp expression: Ksp=[Pb2+][N3−]2 2.5×10−9=S(50012S)2 2.5×10−9=S(5001)24S2 2.5×10−9=(5001)24S3
Solve for S3: S3=42.5×10−9×(5001)2 Calculate (5001)2: (5001)2=25010001 Substitute this value into the equation for S3: S3=42.5×10−9×25010001 S3=462525002.5×10−9 S3=15631250.625×10−9 S3=0.015631250625
Now take the cube root to find S: S=30.015631250625 S=0.250070015...
Rounding to a suitable number of significant figures (given Ksp and Ka have 2 significant figures, and pH has 2 decimal places implying 2 significant figures for concentration), we can round to 2 significant figures. S≈0.25molL−1.
