Question
Question: The molar heat capacity of ethanol is \[{\text{110}}{\text{.4J/K}}{\text{.}}\]. What is its specific...
The molar heat capacity of ethanol is 110.4J/K.. What is its specific heat capacity?
Solution
Specific Heat capacity of any compound or element depends on the Molar heat Capacity and molar mass of the given substance.
Complete step by step answer:
The specific heat capacity, (symbol Cp) is an amount of or the physical measure of the ability of a substance to absorb heat. It is basically the amount of energy that must be added, in the form of heat, to one unit of mass of the substance in order to cause an increase of one unit in its temperature.
Specific Heat is also an intensive property of a substance. Intensive properties are properties of the given substance that are not dependent on the amount of substance present. Some other examples of intensive properties are density, colour, etc
Getting back to our question, as discussed earlier, the specific heat of a substance is dependent on: 1. Molar heat capacity and 2. molar mass
If we were to represent this relation in the form of a formula, it would be:
$$$Cp;=;$ nCmol
Where Cp = specific heat capacity
Cmol= Molar heat capacity
n = molar mass
Now, to calculate the molar mass of ethanol:
No. of Moles of ethanol = molecular weight of ethanolWeight of the given sample of ethanol
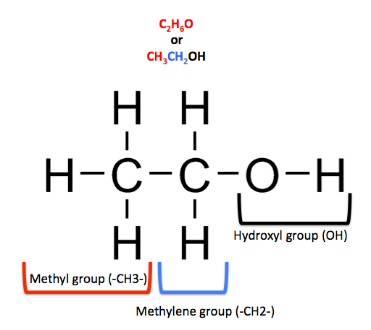
mol. weight of ethanol
= 2(atomic weight of carbon) +6(atomic weight of hydrogen)+1(atomic weight of oxygen) n = 2(12) +6(1) +1(16) n = 46 gm/molSubstituting the value of ‘n’ from and above and the given value of molar heat capacity of ethanol = 110.4J/K; in the previously mentioned formula for calculating specific heat capacity, we get
Cp = 46110.4=2.4JK−1mol−1
Hence, the specific heat capacity of ethanol is 2.4JK−1mol−1
Note:
Specific Heat Capacity and Heat Capacity are two different things. Specific heat capacity is a measure of the amount of heat necessary to raise the temperature of one gram of a pure substance by one-degree K.; while Heat capacity is the ratio of the amount of heat energy transferred to an object to the resulting increase in its temperature
