Question
Question: The molar conductivities $\Lambda_m^\circ$ (NaOAc) and $\Lambda_m^\circ$ (HCl) at infinite dilution ...
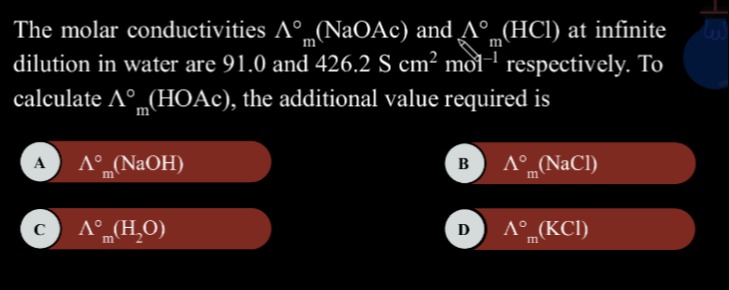
The molar conductivities Λm∘ (NaOAc) and Λm∘ (HCl) at infinite dilution in water are 91.0 and 426.2 S cm2 mol−1 respectively. To calculate Λm∘ (HOAc), the additional value required is
Λm∘ (H2O)
Λm∘ (NaCl)
Λm∘ (NaOH)
Λm∘ (NaNO3)
Λm∘ (NaCl)
Solution
According to Kohlrausch's Law, the molar conductivity at infinite dilution of an electrolyte is the sum of the limiting molar ionic conductivities of its constituent ions.
We are given: Λm∘(NaOAc) = λm∘(Na+) + λm∘(OAc−) Λm∘(HCl) = λm∘(H+) + λm∘(Cl−)
We want to find Λm∘(HOAc) = λm∘(H+) + λm∘(OAc−).
Adding the given equations: Λm∘(NaOAc) + Λm∘(HCl) = λm∘(Na+) + λm∘(OAc−) + λm∘(H+) + λm∘(Cl−)
To obtain Λm∘(HOAc), we need to subtract λm∘(Na+) + λm∘(Cl−), which is Λm∘(NaCl).
Therefore: Λm∘(HOAc) = Λm∘(NaOAc) + Λm∘(HCl) - Λm∘(NaCl)
The additional value required is Λm∘(NaCl).
