Question
Question: The minimum no. of isomers (including stereoisomers) that are possible on monochlorination of the fo...
The minimum no. of isomers (including stereoisomers) that are possible on monochlorination of the following compound:-
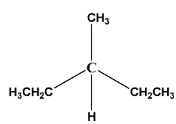
Solution
As we know, monochlorination is a process of replacing a hydrogen atom by a chlorine atom one at a time. Therefore, we must attempt this question by making the entire possible but different monochlorination products of the given compound and then consider each of them, to check whether it has a chiral centre or not so as to achieve all the possible isomers including stereoisomers.
Complete step-by-step answer: First of all let us discuss about the monochlorination process:-
It is a halogenations reaction using chlorine. The process of introducing a chlorine atom in a hydrocarbon by replacing a single hydrogen atom is known as Monochlorination.
So let’s begin with the monochlorination process but before that always remember to check different types of hydrogen atoms present (different with respect to their surroundings). Thereby, always try to write an expanded form of the given compound before doing any further process.
-Expanded form of the given compound:-
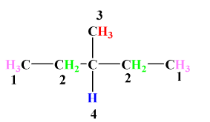
As we can see here, there are 4 different kinds of hydrogen atoms present (marked by different colors) and this takes you to the conclusion that no. of possible product will be 4.
-Monochlorination of the given compound:-
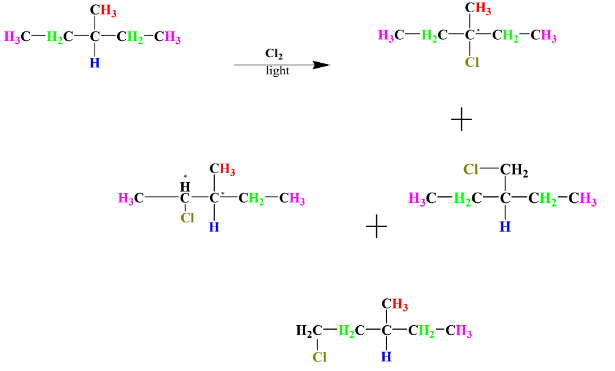
From the above reaction we can see that all the products formed are structural isomers of each other. (Structural isomers are molecules that have the same molecular formulas but different structures. These are also known as constitutional isomers). Therefore, the total no. of structural isomers is 4.
Now let’s discuss about chiral centre and check whether each of the products has chiral centre or not:-
Chiral centre is a centre where a tetrahedral atom has 4 different substituents (atom, group or ion) attached to it. Chiral centres of carbon atoms are also known as asymmetric carbon atoms. If a chiral centre is present in a compound then, it leads to the formation of a pair of enantiomers which are non-superimposable images of each other and thus, count as stereoisomers.
1.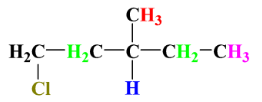
As we can see, there is no chiral centre in this compound as each carbon atom has at least 2 same substituent attached to it. Therefore, it has no stereoisomer present.
2.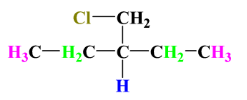
Once again we can see that there is no chiral centre present because each carbon atom is surrounded by at least 2 same groups or atoms. Therefore, it also does not have any stereoisomers.
3.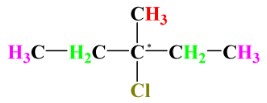
Now, this is the case of chirality. As we can see that the 3rd carbon atom is surrounded by 4 different substituent and hence become the chiral centre. 1 chiral centre gives us 1 enantiomeric pair i.e., 2 stereoisomers.
4.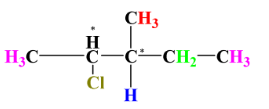
This is the case of chirality. As we can see that the 2nd and 3rd carbon atom is surrounded by 4 different substituents and hence become the chiral centers. 1 chiral centre gives us 1 enantiomeric pair and since we have 2 chiral centres which means 2 enantiomeric pairs i.e., 4 stereoisomers.
Since all these isomers are different from each other. Therefore the total no. of isomers (including stereoisomers) is: 1+1+2+4 = 8.
Note: While solving the isomeric questions always remember the following points:-
-Always try to make an expanded structure of the given compound and check the different possible hydrogen atoms (with respect to its surroundings).
-All the products formed in monochlorination reactions are structural isomers of each other. Check each and every product whether it has a chiral centre or not. If the chiral centre is present, then it will give you stereoisomers as well.
-If there is ‘n’ no. of chiral centre present in a huge unsymmetrical compound, then you can use the formula:-
Total no. of stereoisomer of a molecule or compound = 2
