Question
Question: The minimum energy required to overcome the attractive forces between an electron and the surface of...
The minimum energy required to overcome the attractive forces between an electron and the surface of Ag metal is 552×10−19J. What will be the maximum kinetic energy of electron ejected out from Ag which is being exposed to UV light of λ=360A∘
Solution
When an electron attempts to escape from a metal surface it leaves a positive picture behind on the metal surface. The negative electron switches back to the metal surface due to the attractiveness of this positive image, so it does not permanently leave the metal crystal. But an electron requires adequate energy supplied from outside, typically from external light sources, to overcome the attraction factor. The total energy absorbed by the electron will be the net amount of both energy required to overcome the attractive forces and kinetic energy of the electron.
Formula used:
E=λhC where,
E=energy absorbed from the light
h=6.626×10−34Js which is planck's constant
C=3×108m/s which is the velocity of light
Complete step by step answer:
The given values are,
Energy required to overcome the attractive force (E1)=552×10−19J
The wavelength of light λ=360A∘ which is equal to 360×10−10m
By Substituting the values in equation E=λhC
E=360×10−10m6.626×10−34J×3×108m/s
=5.522×10−18J
We know that here E is the Etotal which is the sum of both energy to overcome attractive forces (E1) and kinetic energy of electrons (Ek)
So Etotal=E1+Ek
Therefore, Ek=Etotal−E1
=(5.52×10−18)−(552×10−19)
=−4.968×10−17J
This is the required maximum kinetic energy of an electron.
Additional information:
The minimum energy for an electron to overcome the attractive forces and to escape from the metal surface by the striking of a photon is called its work function and is denoted by the symbol ϕ.The minimum frequency for this emission is called threshold frequency. Energy in always directly proportional to frequency
E=hν where E is the energy , h is the planck's constant and ν is the frequency. The relation is shown by the following graph.
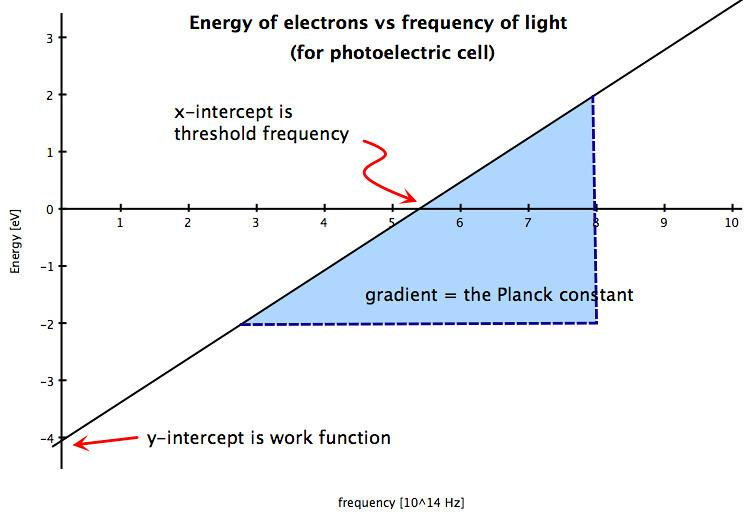
Note:
The energy to overcome the surface is given in J, so while doing the calculations take Planck's constant value also in joule. And wavelengths should need to convert into meters since we are taking the value of velocity of light in m/s.
