Question
Question: The metallic lustre exhibited by sodium is explained by: A.Diffusion of sodium ions B.Oscillatio...
The metallic lustre exhibited by sodium is explained by:
A.Diffusion of sodium ions
B.Oscillations of loose electrons
C.Excitation of free protons
D.Existence of Body centred cubic lattice.
Solution
Sodium is an alkali metal and all metals have a lustrous surface. It is one of their properties. The atoms in a metallic element are bonded to each other by metallic bonding and these atoms consist of free electrons that can absorb energy to get excited to the higher energy levels.
Complete Step by step answer:
Sodium has atomic number 23 and its electronic configuration is: 1s22s22p63s1. The one lone electron is the valence shell of sodium and is readily released by it to form the sodium cation. There are so many such free electrons in the sodium metallic structure and whenever any such free electron absorbs energy to get excited to the higher energy orbitals, they absorb the light of the wavelength that matches with difference in energy of these orbitals. These electrons when they return back to the ground state, release the energy in the form of radiation which causes the lustre on the surface of the metals.
Hence, the correct option is option B.
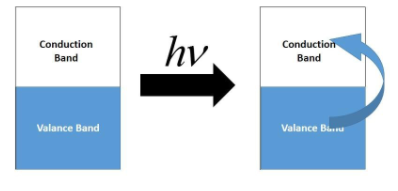
Note: Due to metallic bonding, the filled and the unfilled orbitals in a metal are categorized into two categories; the valence band and the conduction band. The valence band consists of the electrons and the conduction band is empty. When light of a suitable wavelength is absorbed by the surface of the metal, then an electron from the valence band is ejected to the conduction band. This electron when it falls into the valence band again, then the energy released is shown on the surface.