Question
Question: The metal ion (M²⁺) in the following reaction is: $M^{2+} + S^{2-} \rightarrow$ black precipitate $...
The metal ion (M²⁺) in the following reaction is:
M2++S2−→ black precipitate dil.HNO3Δ white precipitate
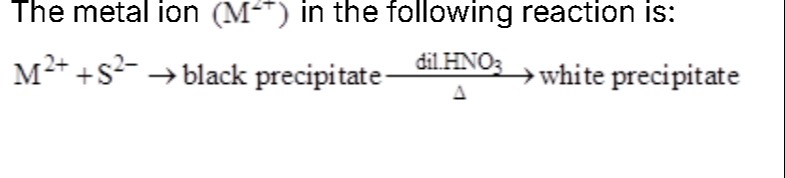
Answer
Pb²⁺ (Lead ion)
Explanation
Solution
When an aqueous solution of a metal ion (M²⁺) is treated with sulfide ions, a metal sulfide precipitate is formed. Lead(II) sulfide (PbS) is black in color. A classical confirmatory test for Pb²⁺ involves forming a black precipitate (PbS) which, upon treatment with dilute nitric acid, converts to a white precipitate (due to formation of a lead salt).
Reaction:
Pb2++S2−→PbS (black precipitate)On heating with dilute HNO3, PbS is converted to a white lead salt, confirming the presence of Pb²⁺.
