Question
Question: The metal d-orbitals that are directly facing the ligands in \({{K}_{3}}\left[ Co{{\left( CN \right)...
The metal d-orbitals that are directly facing the ligands in K3[Co(CN)6] are:
A. dxz,dyx and dz2
B. dxy,dxz and dyz
C. dxy and dx2−y2
D. dx2−y2 and dz2
Solution
. The d-orbitals of the central metal in coordination complexes are going to split into eg and t2g orbitals because of the interaction of the d-orbitals of the central metal with the orbitals of the ligands.
Complete step by step answer:
- In the question it is given that the d-orbitals that are directly facing the ligands in K3[Co(CN)6] .
- We have to find the d-orbitals of the central metal atom that are in the direction of the cyanide (CN) ligands.
- The given complex is an example of an octahedral because the given complex contains six cyanide ligands in its structure.
- We know that cyanide is a strong ligand.
- In octahedral complexes the ligands are going to approach the central metal atom along the axis.
- Then the d-orbitals along the axis are going to face the ligands.
- Now we have to find the d-orbitals which are along the axis.
- The structure of the d-orbitals is as follows.
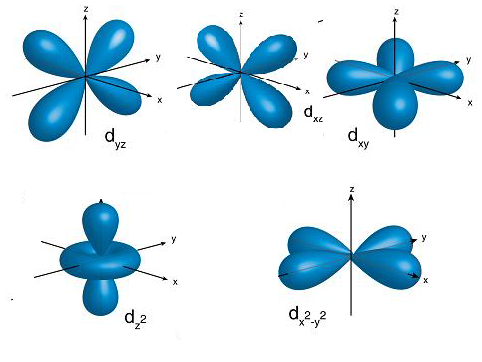
- From the above structures of d-orbitals we can say that the orbitals dx2−y2 and dz2 are along the axis.
- Therefore the metal d-orbitals metal d-orbitals that are directly facing the ligands in K3[Co(CN)6] are dx2−y2 and dz2.
So, the correct answer is “Option D”.
Note: We have to know the structure of the given complex then only we can find the metal d-orbitals which are in the direction of ligands and metal d-orbitals which are not the direction of ligands. In a square planar complex the metal d-orbitals are not in the direction of ligands.
