Question
Question: The maximum number of hydrogen bonds that a molecule of water can have is: (A) \( 1 \) (B) \( 2...
The maximum number of hydrogen bonds that a molecule of water can have is:
(A) 1
(B) 2
(C) 3
(D) 4
Solution
Hint : We have to see the details about hydrogen bonds. We also have to know how hydrogen bonds are formed then we can conclude how many hydrogen bonds can be formed by a molecule of water.
Complete Step By Step Answer:
Hydrogen holding is an exceptional kind of dipole-dipole attraction between particles, not a covalent bond to a hydrogen atom. It results from the appealing power between hydrogen molecules covalently attached to an electronegative particle, for example, an N , O , or F atoms and another electronegative particle. Hydrogen bond strengths range from 4 kJ to 50 kJ per mole of hydrogen bonds.
Highly polar covalent bond forms in those molecules that contain N−H , O−H , F−H bonds. The main reason for forming this bond is the difference in electronegativity between H atom and N , O and F atoms. This difference in electronegativity leads to formation of large partial positive charge in H atom and a large partial negative charge in N , O and F atoms.
A H atom in one molecule is electrostatically attracted to the N , O and F atom in another molecule. Hydrogen bonding between two water ( H2O ) molecules. We can see that the O atom in one molecule is attracted to a H atom in the second molecule. Hydrogen bonding between a water molecule and an ammonia ( NH3 ) molecule. We can see that the N atom in the NH3 molecule is attracted to a H atom in the H2O molecule.
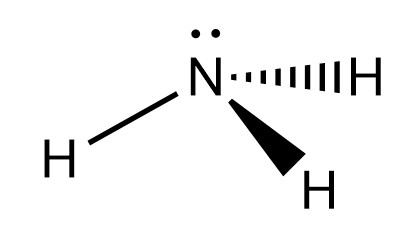
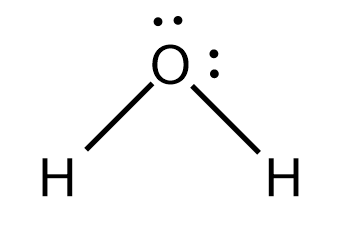
So, if we see the case of water we can conclude that a single water molecule can participate in a maximum of four hydrogen bonds because it can accept two bonds using the lone pairs on oxygen and donate two hydrogen atoms.
Note :
Hydrogen bond is also dependent on temperature. On increasing the temperature the number of hydrogen bonds can be decreased. So, for normal ice, the number of hydrogen bonds is four but for liquid it may be less than that.
