Question
Question: The maximum kinetic energy of the emitted frequency \[\nu \] of incident radiation is plotted as sho...
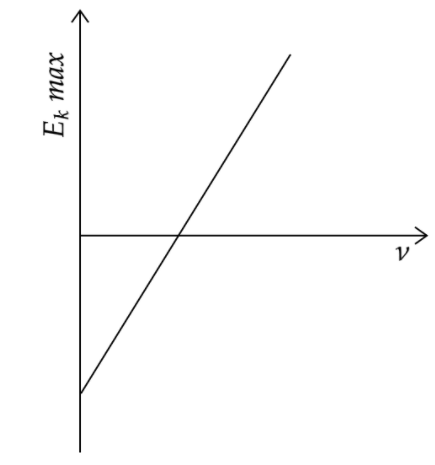
The maximum kinetic energy of the emitted frequency ν of incident radiation is plotted as shown in the fig. This graph helps us in determining the following physical quantities:
(This question has multiple correct options)
A) Work function of the cathode metal
B) Threshold frequency
C) Planck’s constant
D) Charge on an electron
Solution
Before analysing the given graph, we should discuss in brief about the photoelectric effect. The photoelectric effect is the emission of electrons when electromagnetic radiation, such as light, hits a material. Electrons emitted in this manner are called photoelectrons. In classical electromagnetic theory, the photoelectric effect would be attributed to the transfer of energy from the continuous light waves to an electron.
Formula Used:
Ekmax=hν−W
Complete step by step solution:
The Einstein’s equation for photoelectric effect is given by Ekmax=hν−W where Ekmax is the maximum kinetic energy of the photoelectron, h is the Planck’s constant, ν is the frequency of the incident light and W is the work function of the metal. Since Ekmax is plotted along the y-axis and ν is plotted along the x-axis, we can write the equation of the graphical line as y=hx−W.
Comparing the above equation with the standard equation for a straight line y=mx+c , we can say that the slope of the line is given by h , that is the Planck’s constant, the intercept on the y-axis is given by W or the work function of the metal and the intercept on the x-axis gives the threshold frequency needed for photoelectron emission.
Now, we can analyse the options and say that options (A), (B) and (C) are the correct answers or the graph of the photoelectric effect helps in the determination of the Work function of the cathode metal, the threshold frequency and the Planck’s constant.
Note: It is a universally known fact that the slope of the graphical curve of the photoelectric effect gives the value of the Planck’s constant. So sometimes students only choose the option of the Planck’s constant and they move forward. It is, therefore, always advised to check all the possible options as sometimes multiple options are correct and the examiner doesn’t mention this fact to check the wit of the students.
