Question
Question: The maximum distance between interatomic lattice planes is \(15\;A^{\circ}\). The maximum wavelength...
The maximum distance between interatomic lattice planes is 15A∘. The maximum wavelength of x-rays which are diffracted by this crystal will be:
A. 15A∘
B. 20A∘
C. 30A∘
D. 45A∘
Solution
We need to arrive at an expression that relates the distance between the lattice planes and the wavelength of light incident on them. For this, consider two rays striking two adjacent lattice planes and determine the difference in the path length travelled by the two rays, as the ray hitting a lower plane travels more distance. We know that for constructive interference, the difference in path length is generally an integral multiple of the wavelength of incident light. Equate the two expressions thus obtained to arrive at the required expression, and plug in the given values. Remember to use the upper limit for quantities in the resultant equation whose values are not explicitly mentioned since we have to find the maximum wavelength.
Formula used:
Bragg’s Law: 2dsinθ=nλ
Complete step-by-step answer:
Let us begin by establishing a relation between the distance between interatomic lattice planes and the wavelength of light incident on them.
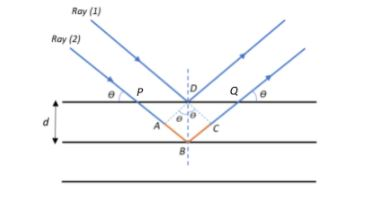
Given that x-rays of wavelength, say λ are incident on a crystal as shown in the figure, consider two rays, Ray 1 and Ray 2, striking two adjacent planes that are at a distanced apart. Let the rays make an angle θ with the vertical as they strike the lattice planes. We see that Ray 2 travels more distance to hit the inner plane than Ray 1 that hits the closest plane. We shall now find the path difference between the two rays.
From the diagram, we see that the path difference is ΔP=AB+BC.
We see that ∠APD=θ and ∠DAP=90∘, and from ΔAPD, we have:
∠ADP=180−(90+θ)=90−θ (Since the sum of all angles in a Δ=180∘)
Now, from the figure, since ∠PDB=90∘ and ∠ADP=90+θ,
∠ADB=∠PDB−∠ADP=90–(90−θ)=θ
Therefore, from right angled ΔADB:
sinθ=DBAB⇒AB=DBsinθ=dsinθ
Similarly, BC=dsinθ, therefore, path difference:
ΔP=AB+BC=dsinθ+dsinθ=2dsinθ
Now, for constructive interference, the difference in path length is an integral number of the wavelength of incident light, i.e.,
ΔP=nλ, where n denotes the order of diffraction that is usually set to unity.
Equating the above two expressions, we get:
nλ=2dsinθ
This is nothing but Bragg’s law for constructive interference.
⇒λ=n2dsinθ
Now, we are given that the maximum distance between the lattice planes in dmax=15A∘=15×10−10m. Therefore, the maximum wavelength of x-rays which are diffracted by this crystal will be:
λmax=nmax2dmaxsinθmax
We know that the intensity maxima is obtained at nmax=1, and the maximum value of sinθ will be at θmax=90∘, since sin90∘=1. Plugging this into our wavelength expression, we get:
λmax=12×15×10−10×1=30×10−10m=30A∘
So, the correct answer is “Option C”.
Note: X-ray diffraction is a technique that is generally used to determine the crystallographic structure of a material by observing the diffraction pattern thus obtained when x-rays are made incident on a crystal. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal and consequently determine the nature of the crystal. Thus, it is important to note that the x-rays impinging on the crystals get diffracted not by the planes themselves but by the constituents of the planes, which are usually electrons arranged in regular arrays within the crystal structure.
