Question
Question: The maximum covalency of oxygen in ozone is : A.\(5\) B.\(3\) C.\(2\) D.\(4\)...
The maximum covalency of oxygen in ozone is :
A.5
B.3
C.2
D.4
Solution
In any compound , elements share their electrons with other atoms of the same or different element to obtain stable electronic configuration . The number of electrons shared by the elements to get stable configuration is known as covalency.
Complete step by step answer:
Ozone is a form of elemental oxygen. It is a very unstable form of oxygen. Most stable form of oxygen is its diatomic molecule i.e. O2 . It is found naturally in the Earth’s upper atmosphere.
Ozone is also known as tri oxygen which is an inorganic molecule and its colour is pale blue and possesses a pungent smell. It is a lot of oxygen.
Structural formula of compound is given the figure: Central oxygen atom i.e. O+ shares its three electrons and other two atoms share two and one electron respectively . Hence maximum covalency of oxygen in ozone is three.
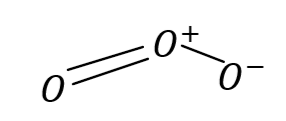
So, the correct answer is Option B .
Note:
Ozone saves us from the harmful UV rays of the sun . Ozone also shows resonance and it has two resonating structures, they contribute equally to the hybrid structure . Boiling point of ozone is −112∘C and it is soluble in water , sulphuric acid and carbon tetrachloride . Ozone is harmful for humans as it can damage the lungs, it can cause chest pain , coughing and throat irritation . It is found in the stratosphere which is the second layer of the Earth’s atmosphere as it protects human beings from harmful UV rays.
