Question
Question: The mass of \[10\% \] glucose solution is 200g. Find the masses of the solute and the solvent used t...
The mass of 10% glucose solution is 200g. Find the masses of the solute and the solvent used to prepare the solution.
Solution
Glucose is one of the most important carbohydrates having the structural formula C6H12O6 . Its simplest or empirical formula comes out to be CH2O . The empirical formula of carbohydrate comes from the fact that a carbohydrate is a biomolecule consists of carbon(C), hydrogen(H), and oxygen(O) atoms, with a hydrogen-oxygen atom ratio of 2:1 and the ratio of carbon to hydrogen to oxygen is 1:2:1
Formula used: %(mm)=massofsolutionmassofsolute×100%
Complete step by step answer:
The molar mass of glucose solution, C6H12O6 =108.156 g/ml
According to the question, a 10% glucose solution has a mass of 200 gm. Therefore the 10% of 200gm would be the mass of glucose which is the solute and the rest of the mass is the mass of water which is the solvent in this solution.
Therefore, 10% of 200 gm is,
200×10010=massofsolute
=20gm
And rest of the mass of 200 gm is,
200-20
=180gm
Therefore, the mass of solute and solvent of 10% glucose solution with the total mass of 200 is, 2o gm and 180 gm respectively.
Additional information:
We can refer to the molecular formula as carbon water or hydrates of carbon and represent it as (CH2O)n , where n can be any number greater than 3. Therefore, it can also be written as Cn(H2O)n . This formula explains the meaning of the term ‘carbohydrate’ i.e. the components are carbon for carbo and water for hydrate. The simplest carbohydrate thus formed by putting n as 1 is glucose, C6H12O6 .
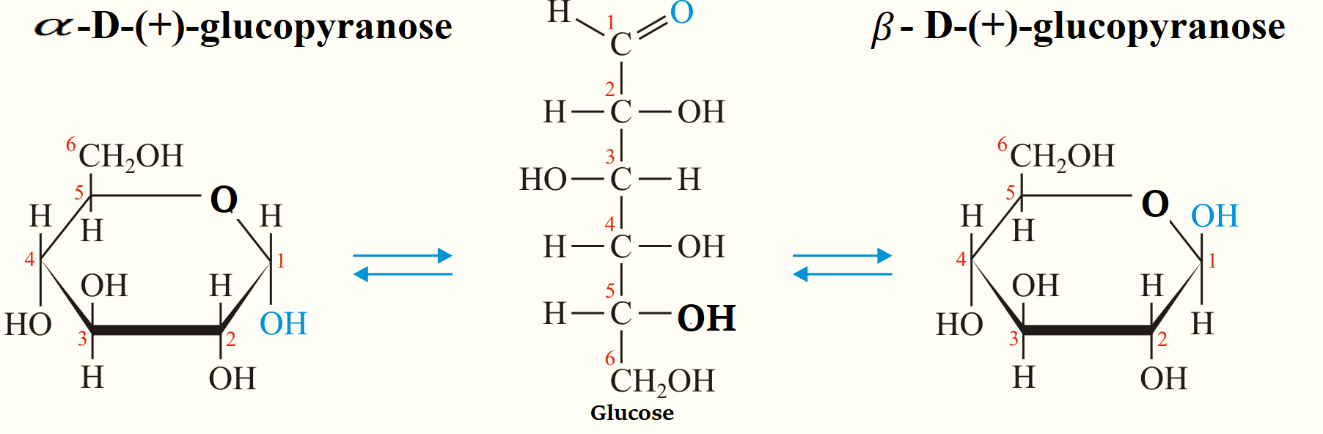
Note: Glucose has two forms which are α−D−(+)− glucopyranose and β−D−(+)− glucopyranose.
To draw the structures of α−D−(+)− glucopyranose and β−D−(+)− glucopyranose, a simple six-membered pyranose ring having five carbon atoms and one oxygen atom is drawn first. These structures were first suggested by Haworth and we call them Haworth projection formulae.
In the pyranose ring, CH2OH the group is added at the terminal, placed above the plane of the hexagon ring always. Groups that are present on the left-hand side in Fischer projection are placed above the plane of the ring and placed all the groups of the right hand below the plane of the ring.