Question
Question: The major products $P_1$ and $P_2$, respectively, in the following reaction sequence are ...
The major products P1 and P2, respectively, in the following reaction sequence are
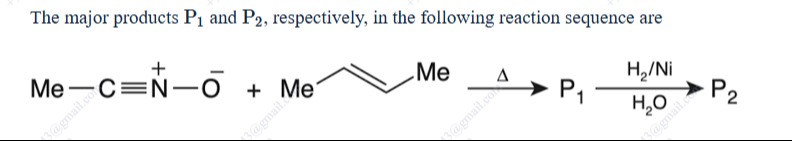
Answer
P₁ = 3,4,5–trimethylisoxazoline P₂ = 2–amino–3,3–dimethyl–1–butanol
Explanation
Solution
- The 1,3–dipolar cycloaddition of methyl cyanide N–oxide [CH₃–C≡N⁺–O⁻] with trans–2–butene (CH₃–CH=CH–CH₃) involves only the dipolar atoms (C–N–O) and the two alkene C’s. Each alkene C still bears its –CH₃ group. Thus, the five–membered adduct is an isoxazoline in which the (nitrile–oxide) C (with CH₃) appears as one substituent and the two alkene carbons carry a CH₃ each.
So, P₁ = 3,4,5–trimethylisoxazoline.
- Catalytic hydrogenation (H₂, Ni, H₂O) breaks the N–O bond and opens the ring in a well–known transformation to give a 1,4–amino alcohol. In our case the opened structure is best drawn as
P₂ = 2–amino–3,3–dimethyl–1–butanol. (A balanced carbon–count: 4–carbon main chain + 2 extra Me groups = 6 C’s overall.)
