Question
Question: The major products of the following reaction are: 
[A] 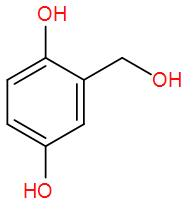 and formic acid
and formic acid
[B] 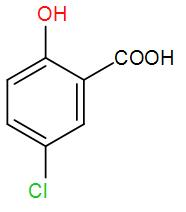 and methanol
and methanol
[C] 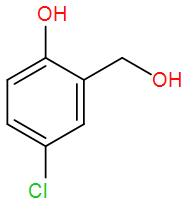 and formic acid
and formic acid
[D] 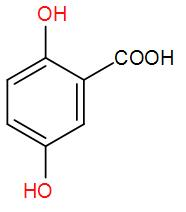 and methanol
and methanol
Solution
. The Reimer - Tiemann reaction is carried out on phenols in presence of chloroform and we obtain an aldehyde as the end product. It proceeds through an electrophilic substitution pathway. You can use this to answer the given question.
Complete step by step answer:
In the question, 4-chlorophenol is given to us. To this, chloroform in an alkaline medium is added. The reaction will proceed via Reimer – Tiemann reaction.
So, firstly let’s discuss the Reimer – Tiemann reaction.
When we treat phenol with chloroform and an aqueous hydroxide, an aldehyde group –CHO is introduced onto the aromatic ring. Generally, the aldehyde is introduced at the ortho-position with respect to the –OH group.
The Riemer-Tiemann synthesis reaction involves electrophilic substitution on the highly reactive phenoxide ring. Here, the electrophilic reagent is dichlorocarbene (:CCl2), which is generated from chloroform through the action of base. Although dichlorocarbene is electrically neutral but it contains carbon with only a sextet of electrons which makes it highly electrophilic-
OH−+CHCl3⇄H2O+−:CCl3→Cl−+:CCl2
Therefore, here we can write the reaction as-
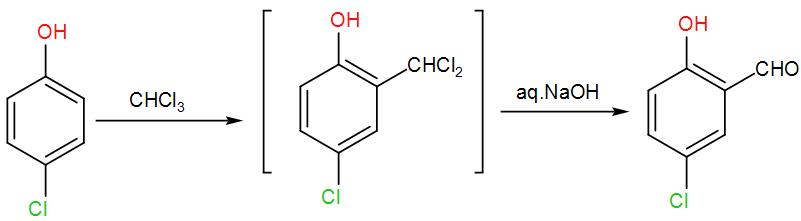
Now to this formaldehyde and concentrated sodium hydroxide is added. Here, the Cannizzaro reaction will take place. Formaldehyde with sodium hydroxide will form methanol and sodium formate. Sodium formate will react with the aldehyde obtained above (4-chlorosalicylaldehyde) and will give alcohol and a carboxylic acid. We can write the reaction as-
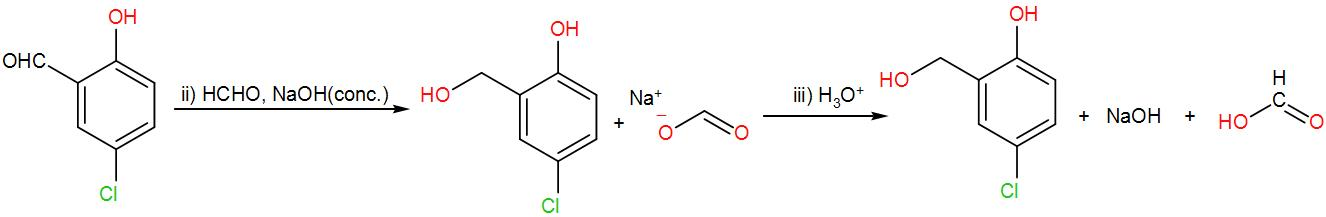
Therefore, we can understand from the above reactions that the correct answer is option –
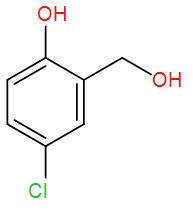 and formic acid
and formic acid
So, the correct answer is “Option C”.
Note: In the Reimer-Tiemann reaction, the phenoxide ion formed will show mesomeric and inductive effect hence, the reaction might take place at ortho or para position. But as we know, +I-effect decreases with increasing distance, therefore the ortho position will be electron rich and the incoming electrophile will attack at the ortho position. Therefore, formylation will take place at the ortho position.
