Question
Question: The major product ‘Y’ in the following reaction is: 
(A) 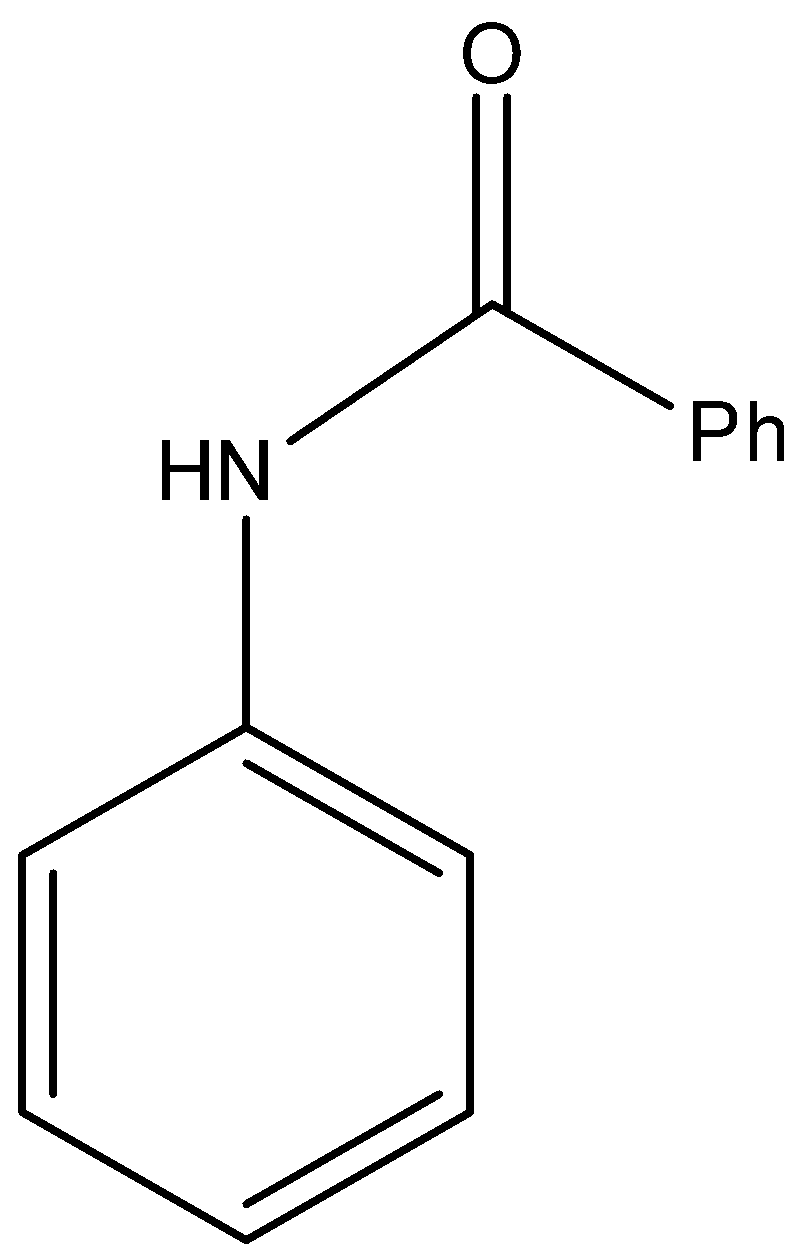
(B) 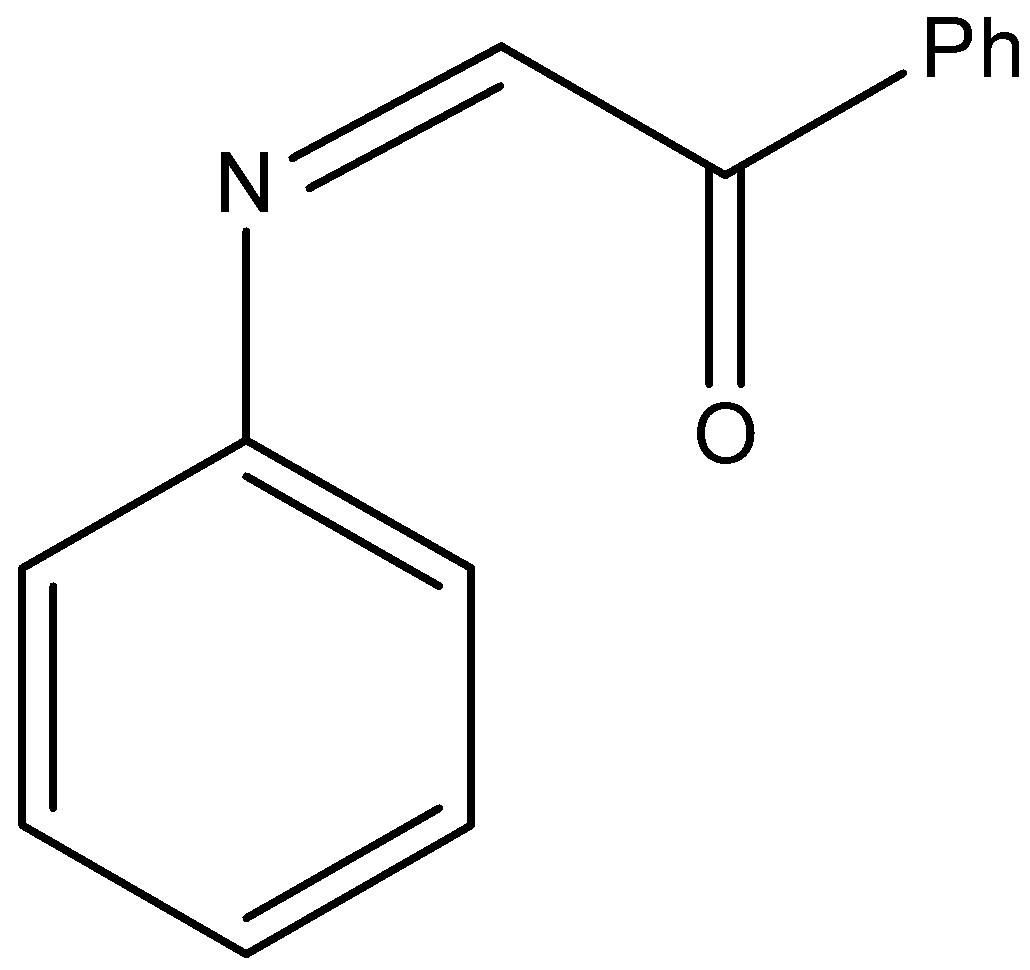
(C) 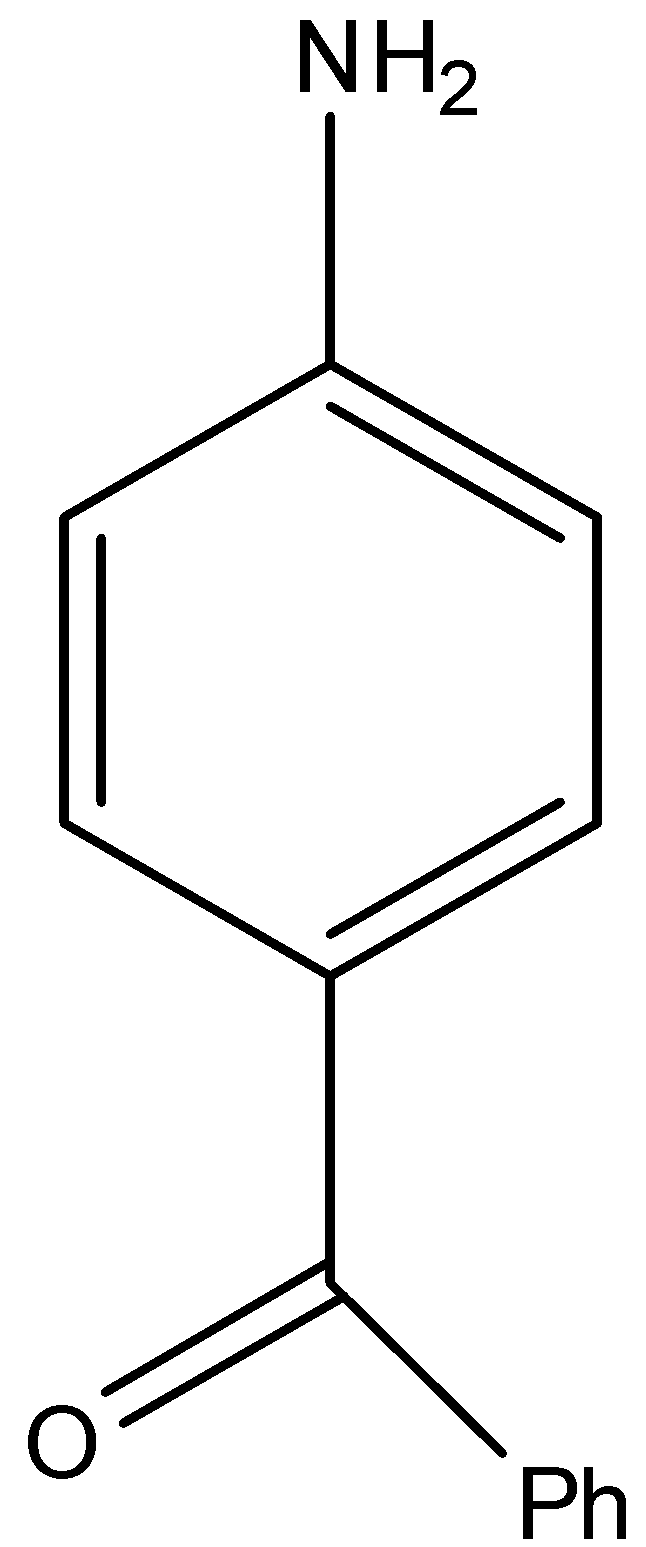
(D) 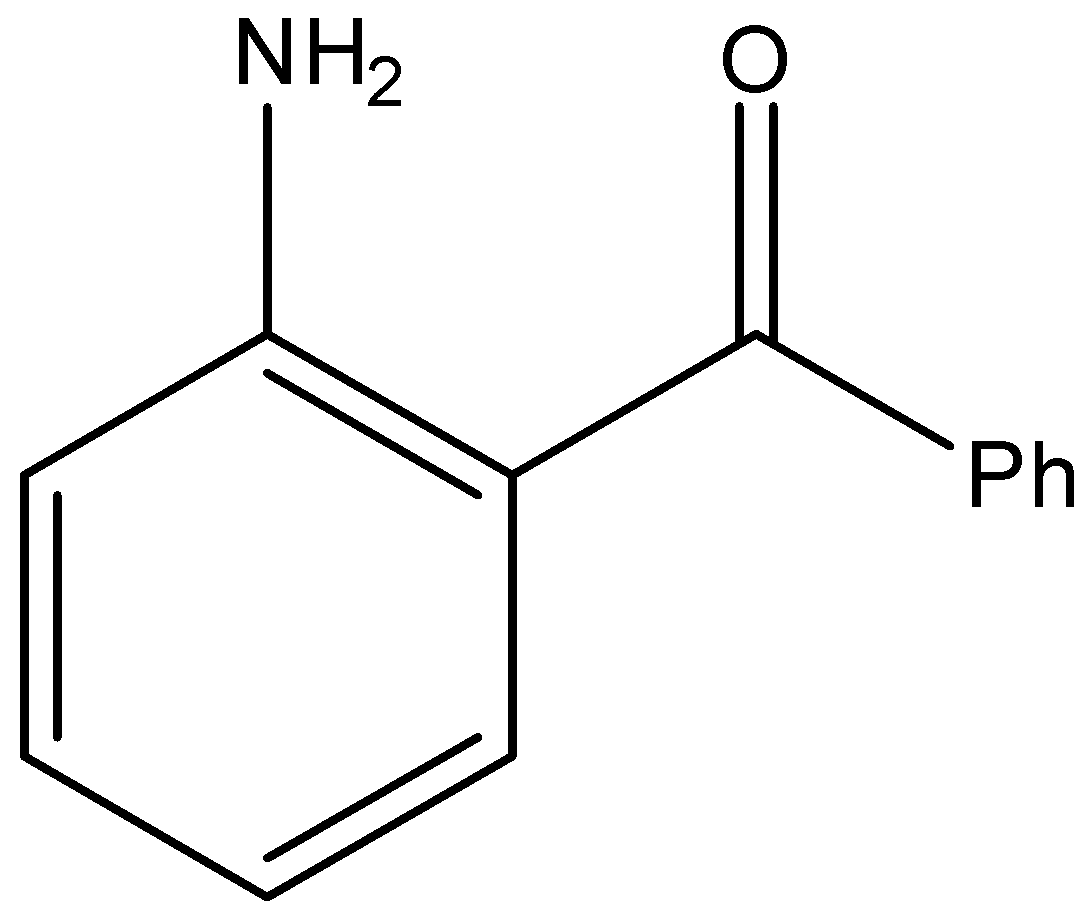
Solution
. Sodium hypochlorite (NaOCl) will give a haloform reaction when it will react with methyl ketones. Thionyl chloride (SOCl2) will give acid chlorides when allowed to react with carboxylic acids. Aniline will give a nucleophilic substitution reaction.
Complete step by step answer:
We will see the reaction step by step with mechanism.
- The substrate is Benzophenone which is allowed to react with sodium hypochlorite. We know that the methyl ketones give a haloform reaction when allowed to react with hypohalite solutions. So, it will have chloroform and a carboxylic acid as a product. The mechanism can be given as below.
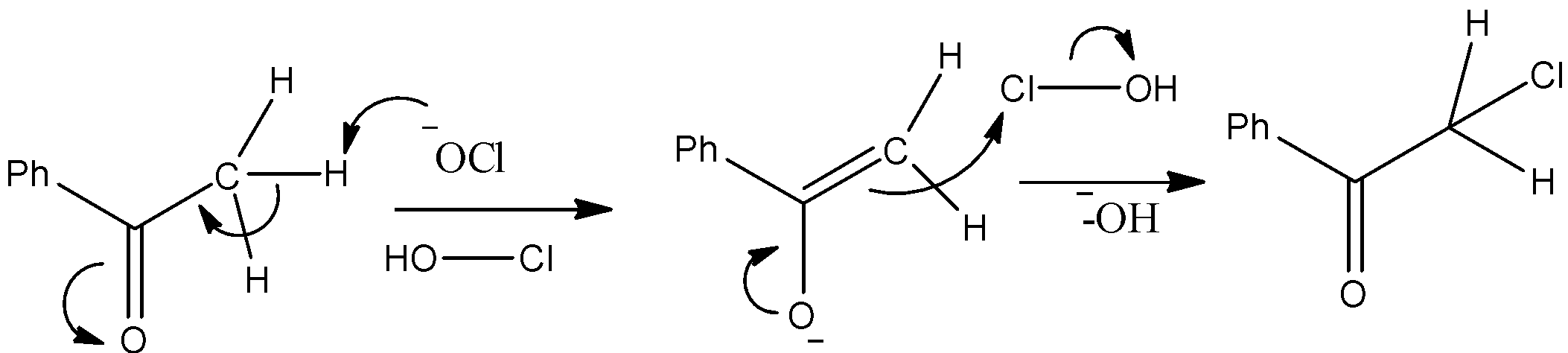
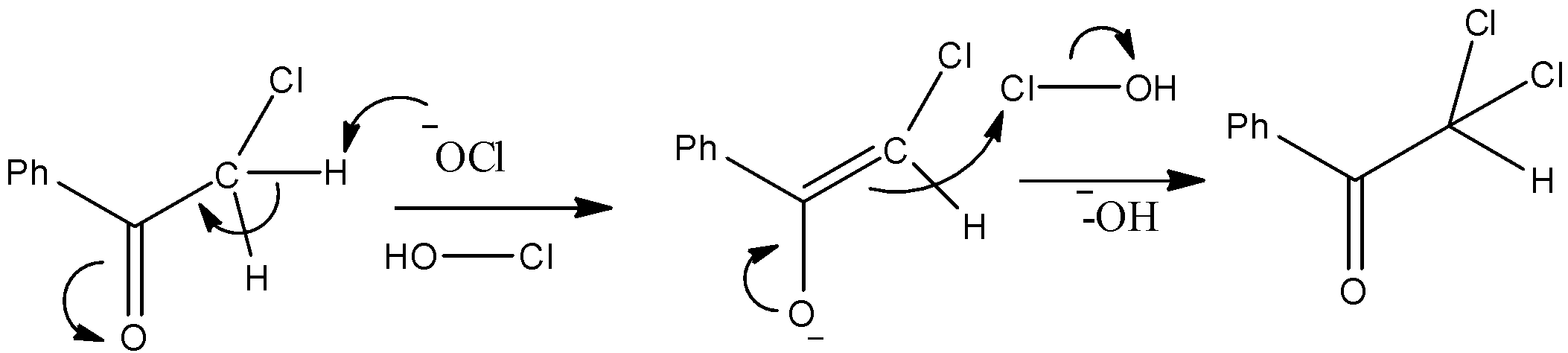
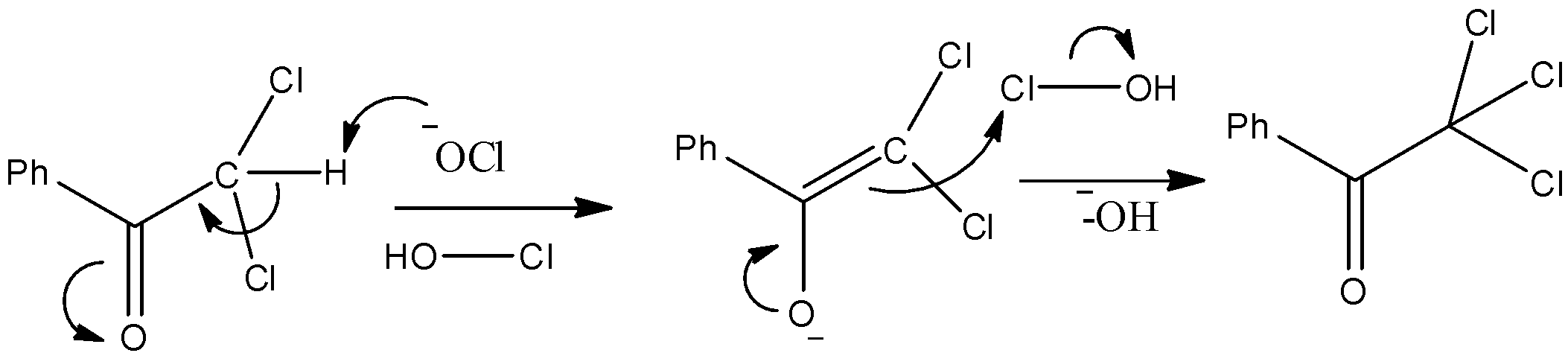
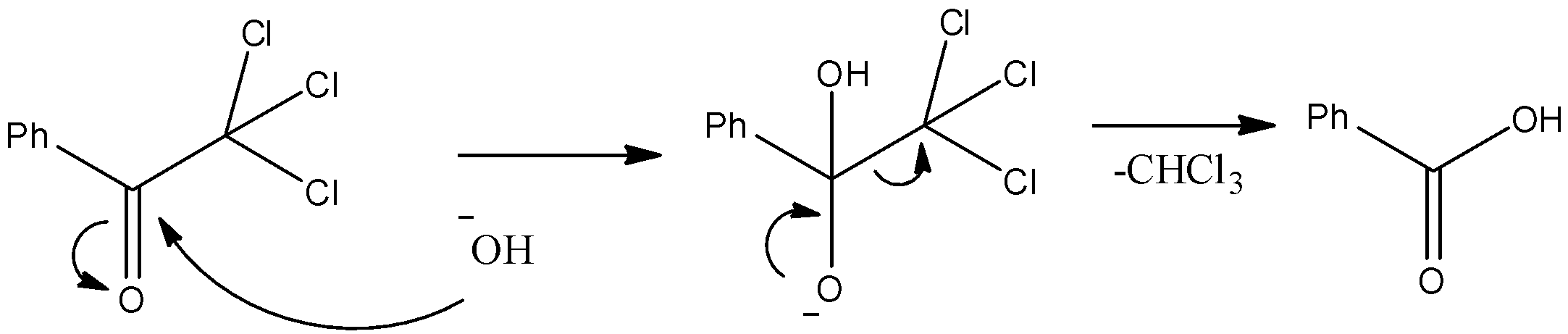
- Thus, we can see that the nucleophilic O atom of ClO− ion attacks the hydrogen atom. Consequently Chloroform gets removed and we obtain benzoic acid which is compound “X” as given in the question.
- Now, we will see the second step of the reaction.
- We know that SOCl2 (Thionyl chloride) is a chlorinating agent. It will form acid chloride upon its reaction with a carboxylic acid functional group. So, it will react with benzoic acid to give the following reaction.
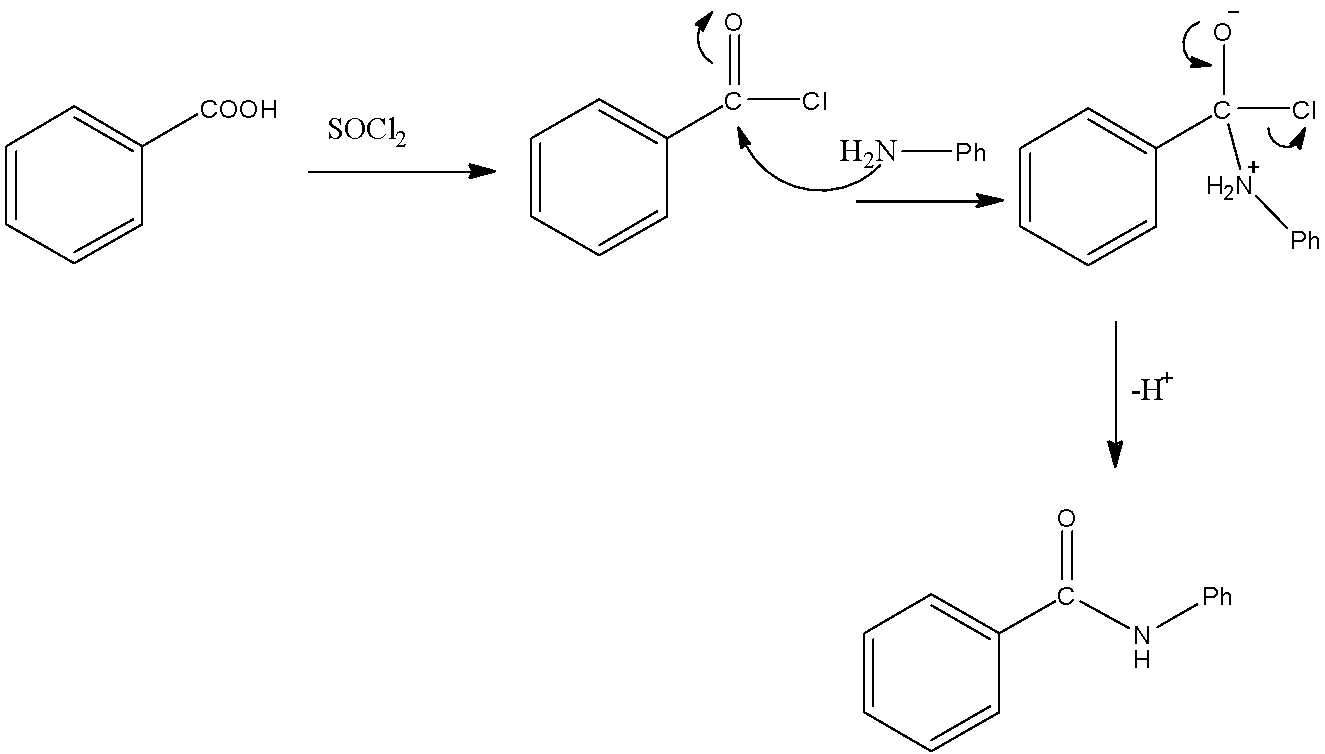
- Here, we can see that as aniline has a nucleophilic nitrogen atom, it will attack the electrophilic carbon atom of the carbonyl group. This will remove the chlorine atom on the carbonyl carbon. As a result, we obtain the final product “Y” as shown in the compound.
So, the correct answer is “Option A”.
Note: Note that hypochlorite ions do not directly attack the carbonyl carbon. Actually, they first attack the hydrogen atoms of the methyl group. Here, benzoic acid would have formed even if the reagent was NaOI in place of NaOCl.
