Question
Question: The major product P of the given reaction is: \(C{H_3} - CH = C{H_2} + HOBr \to P\)....
The major product P of the given reaction is:
CH3−CH=CH2+HOBr→P.
Solution
Markovnikov’s Rule also known as Markownikoff’s rule is used to determine the outcome of some chemical addition reactions. Basically, hydrogen is added to the carbon with the most hydrogens and the halide is added to the carbon with least hydrogens. So, we will apply this rule in the given reaction.
Complete step by step answer:
First of all, let’s discuss Markovnikov's rule. In this, when a protic acid is added to an asymmetric alkene, then the acidic hydrogen attaches itself to the carbon having a greater number of hydrogen substituents whereas the halide group attaches itself to the carbon atom which has a greater number of alkyl substituent.
Now, in the first step, the alkene is protonated and it gives rise to the more stable carbocation. Further, two types of carbocation are formed i.e. primary carbocation and secondary carbocation. We generally prefer secondary carbocation because it is far more stable primary. Then the halide ion i.e. the nucleophile attacks the carbocation. Since, we prefer secondary carbocation so, the major product will be secondary.
So, the addition reaction of hydro bromic acid with propene and the product P is as shown:
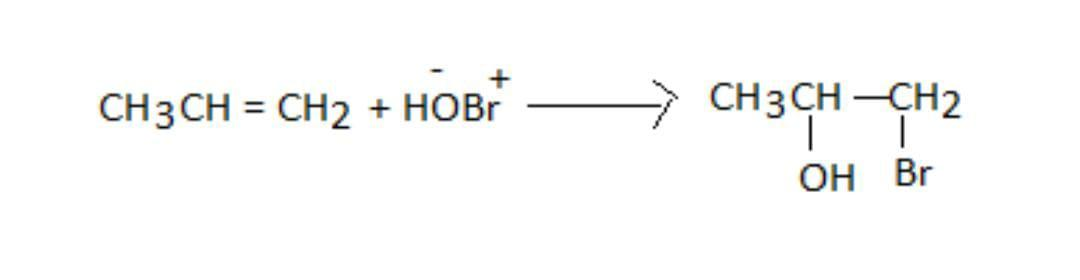
So, we can see that the negative part goes and ads to the carbon bearing the lesser number of hydrogen atoms. As −OH is the negative part, it adds to propene.
Note: The free radical addition reactions do not obey Markovnikov’s rule since the regioselectivity of the mechanism of these reactions are not predicted by this rule. These reactions are generally referred to as Anti-Markovnikov addition reactions.
