Question
Question: The major product P of the following reaction is: ...
The major product P of the following reaction is:
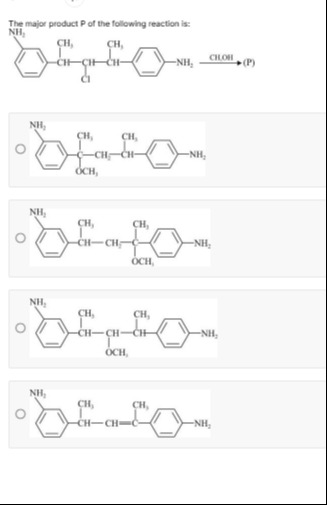
Option 2
Solution
The reaction involves a secondary alkyl chloride reacting with methanol (CH₃OH). Methanol is a protic solvent and a weak nucleophile, characteristic of an S_N1 reaction mechanism, which proceeds via a carbocation intermediate.
-
Formation of Carbocation: The chlorine atom departs, forming a carbocation. A secondary carbocation is formed.
-
Carbocation Rearrangement: A 1,2-hydride shift from Cγ to Cβ forms a tertiary carbocation. This tertiary benzylic carbocation is significantly more stable than the initial secondary benzylic carbocation.
-
Nucleophilic Attack: Methanol (CH₃OH), attacks the more stable tertiary carbocation at Cγ.
-
Deprotonation: A molecule of methanol deprotonates the oxonium ion to yield the final ether product. The major product will have the methoxy group (OCH₃) attached to the carbon that was originally Cγ.
The reaction is an S_N1 reaction. The initial secondary carbocation rearranges via a 1,2-hydride shift to form a more stable tertiary benzylic carbocation, which is further stabilized by the para-amino group. Methanol then acts as a nucleophile, attacking this stable carbocation, followed by deprotonation to yield the ether product. The methoxy group is attached to the carbon that was originally part of the CH(CH₃) group adjacent to the p-aminophenyl ring.
