Question
Question: The major product of the reaction is: 
A.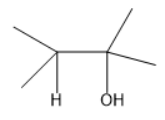
B.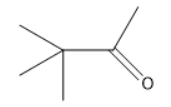
C.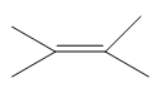
D.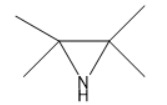
Solution
When primary amines with a hydroxyl group in the same molecule treated with sodium nitrite in presence of sulphuric acid converts an amine to dinitrogen, this dinitrogen will be eliminated and further undergoing methyl shift and proton rearrangement forms a carbonyl compound.
Complete answer: The primary amines are the compounds that nitrogen is linked to only one carbon atom. Given compound has a primary amine as there is a presence of −NH2 group linked to a single carbon atom. The molecule also consists of a hydroxyl group.
When this molecule is treated with sodium nitrite in presence of sulphuric acid. The primary amine converts into the nitrogen and this nitrogen will be eliminated and the carbon gets a positive charge. Later the methyl group from the adjacent carbon will be shifted to this carbon which is known as 1,2 methyl shift. Thus, the carbon attached with the hydroxyl group gets a positive charge and accepts electrons from the oxygen atom in the hydroxyl group thereby forming a positive charge on the oxygen atom. The hydrogen atom donates its electrons to form a carbonyl compound. Here the carbonyl compound is ketone.
The chemical reaction involved will be as follows:
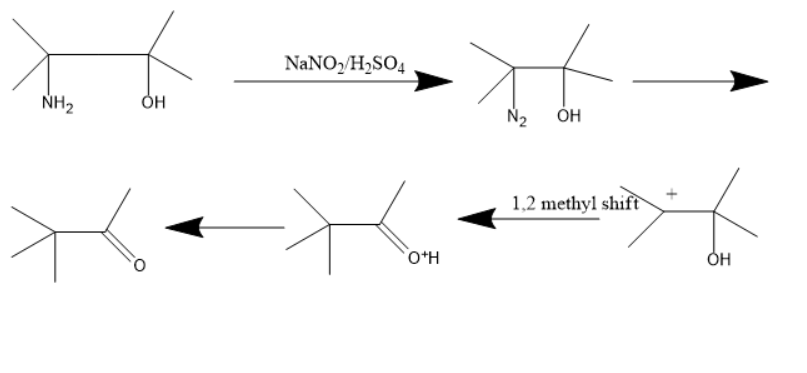
Thus, the product is 2,2 dimethyl 3 one.
The correct answer is Option (B).
Note:
The methyl shift is an important step in the above reaction. Methyl shift generally takes place in so many organic reactions. The valencies of all the above atoms must be correct. As oxygen has a lone pair of electrons it donates to the carbocation, but generally oxygen attracts electrons from carbon.
