Question
Question: The major product of the given reaction is ```latex \begin{array}{c} \text{CH}_3 \\ \text{H}_3\text{...
The major product of the given reaction is
\begin{array}{c} \text{CH}_3 \\ \text{H}_3\text{C}-\text{C}-\text{CH}_3 \\ \text{O}-\text{CH}_2-\text{Ph} \end{array} \xrightarrow[\text{(1 eq.)}]{\text{HI}} \text{Pro}
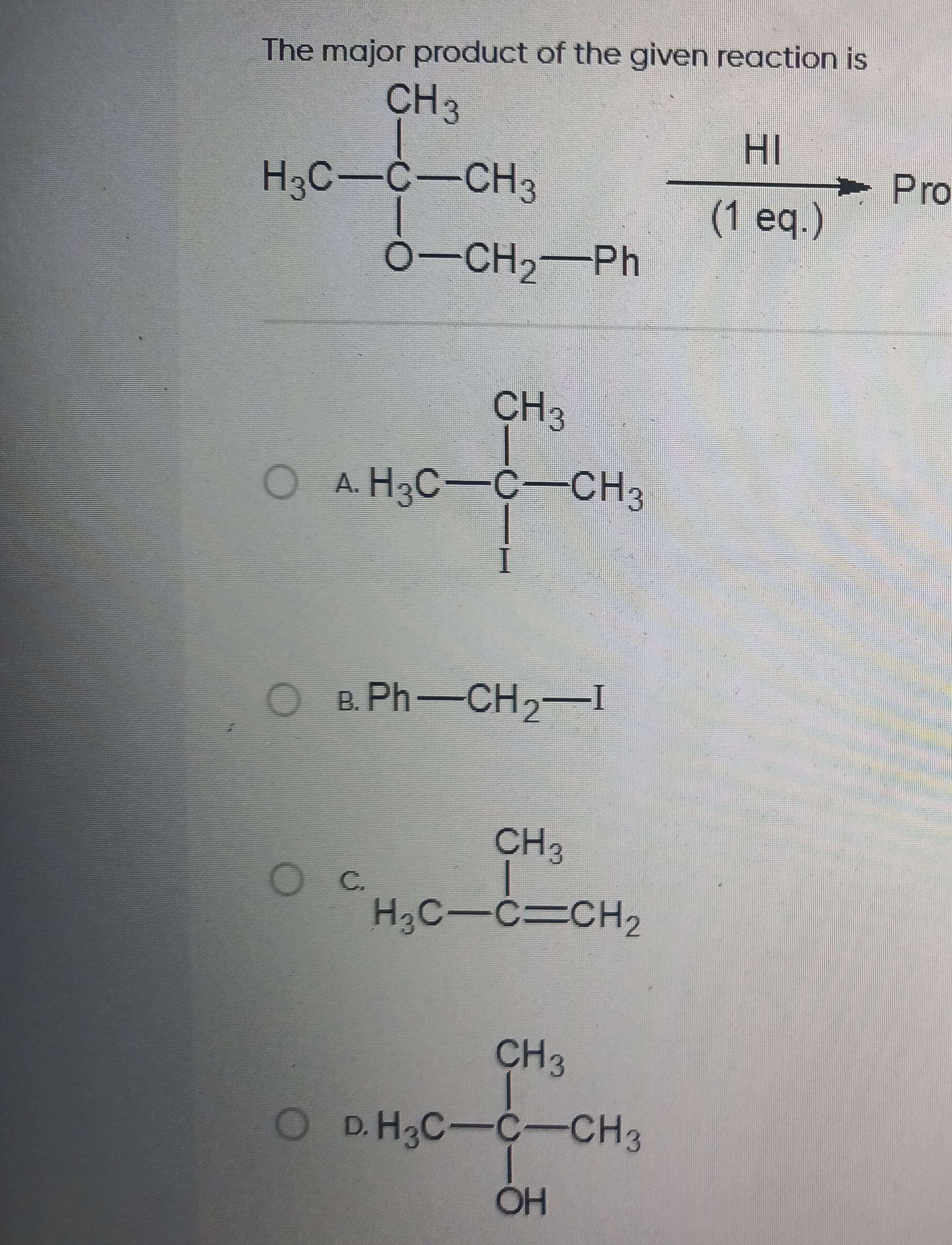
A
\begin{array}{c} \text{CH}_3 \\ \text{H}_3\text{C}-\text{C}-\text{CH}_3 \\ \text{I} \end{array}
B
Ph-CH2-I
C
\begin{array}{c} \text{CH}_3 \\ \text{H}_3\text{C}-\text{C}=\text{CH}_2 \end{array}
D
\begin{array}{c} \text{CH}_3 \\ \text{H}_3\text{C}-\text{C}-\text{CH}_3 \\ \text{OH} \end{array}
Answer
Ph-CH2-I
Explanation
Solution
HI cleaves ethers. The oxygen is protonated, and then the iodide ion attacks the more substituted carbon to form the more stable carbocation. Here, the benzyl carbocation is more stable than the tert-butyl carbocation. Thus, the C-O bond to the benzyl group cleaves, forming benzyl iodide and tert-butyl alcohol. The tert-butyl alcohol reacts further with HI to form tert-butyl iodide. However, the formation of benzyl iodide is kinetically favored due to the stable benzyl carbocation intermediate.
