Question
Question: The major product of the given reaction is: 
(A) 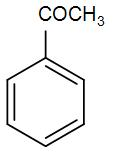
(B) 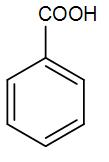
(C) 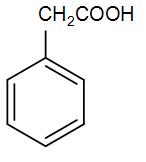
(D) 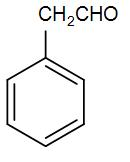
Solution
HINT: Here, the given compound to us is ethyl benzene. To answer this, you must know that potassium permanganate is an oxidising agent. In an alkaline medium, it will oxidise alkane and give a carboxylic acid. You can use this to answer the given question.
COMPLETE STEP BY STEP SOLUTION: Here, the compound given to us is ethyl benzene. Ethyl benzene is treated with potassium permanganate.
-We know that potassium permanganate is a strong oxidising agent i.e. it oxidises ethyl benzene. This reaction takes place in an alkaline medium. Also, we must know that oxidation of alkane gives us acid. However, this reaction only works in the presence of a benzylic carbon is present i.e. a carbon atom is directly attached to the aromatic ring. Now, let us see the reaction that is taking place here.
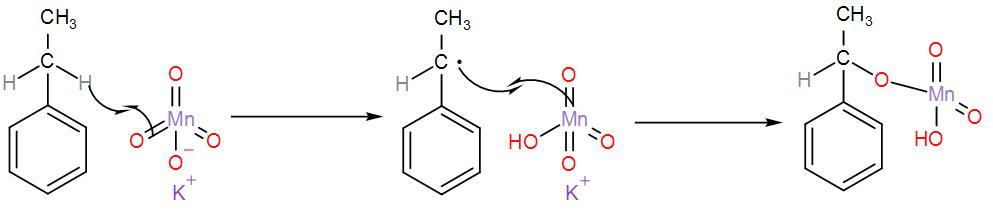
-Here, the proton is abstracted by the KMnO4 in the solution. One proton abstraction is shown above and the other one is abstracted in the same way and the ethyl group is oxidised to carboxylic acid, hence giving us benzoic acid. We can write the reaction as-
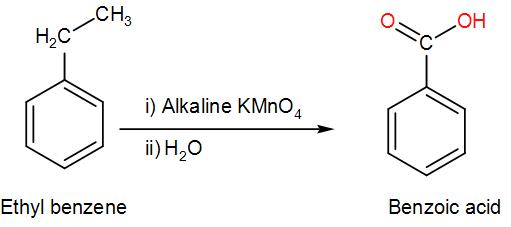
-From the above discussion and reactions, we can understand that the major product obtained in the given reaction is benzoic acid.
Therefore, the correct answer is option [B]
NOTE: Here, the reaction would not take place if there was a tertiary butyl group present instead of the ethyl group. Due to the absence of hydrogen atoms attached to the benzylic carbon, the reaction would not be feasible. We can also carry out the above reaction i.e. formation of benzoic acid from ethyl benzene by other oxidizing agents like potassium dichromate or nitric acid. The reaction would proceed in a similar manner and give us benzoic acid as a product.
