Question
Question: The major product of the given reaction is: 
A. 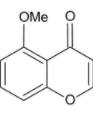
B. 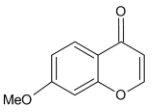
C. 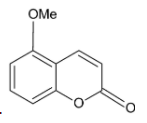
D. 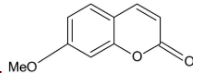
Solution
To answer this question, you must be familiar with reactions of various oxygen containing organic compounds. Acid chlorides are much more reactive than aldehydes, ketones and carboxylic acids.
Complete step by step solution:
We know that acid chlorides are more reactive than aldehydes and thus the acid chloride group will be the one reacting with the phenolic hydroxyl group. The acid chloride and hydroxyl group undergoes an esterification reaction.
We are familiar with the fact that methoxy group is an ortho para directing group. Thus, we can say that the para position (to methoxy group) of the phenyl ring is activated and susceptible to nucleophilic attack.
On treatment with concentrated sulphuric acid, a water molecule is lost and the molecule undergoes cyclisation. A six membered cyclic ring is formed.
We can write the reaction as,
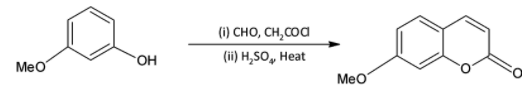
Thus, the correct option is D.
Note:
Acid halides are derived from carboxylic acids by replacing the hydroxyl group in the acid by a halide. A unique property of an acid halide is its inability to form hydrogen bonds with other compounds. This absence of hydrogen bonds affects their boiling and melting point. In comparison, it can be clearly seen that the melting and boiling points of acid halides are significantly lower than those of carboxylic acids.
Acid halides are extremely reactive compounds. Each of their reactions involves the replacement of the halide group by another functional group, that might be the reacting nucleophile. All reactions of acyl halides are characterized by production of steamy acidic fumes of hydrogen halides in the first step.
