Question
Question: The major product of the following reaction sequence is: 
A. 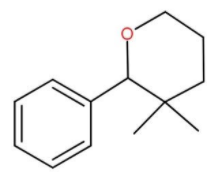
B. 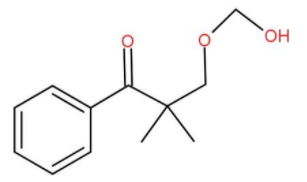
C. 
D. 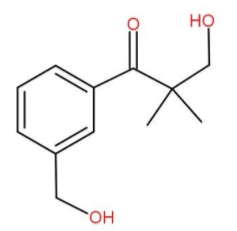
Solution
When two molecules of aldehyde and ketones having α-hydrogen condense together in presence of NaOH to form β-hydroxy aldehyde or β-hydroxy ketone respectively which are collectively called as aldol. This reaction is called aldol condensation.
Complete step by step solution:
We have to compound where one is a ketone group attached to an aryl group and to an isopropyl group.
(i) In first reaction it is treated with formaldehyde in (excess) NaOH, heat
The formaldehyde attacks the αpart of the ketone group. Then we get
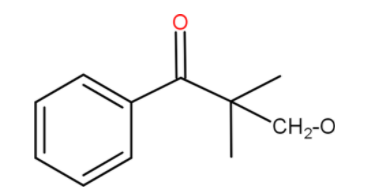
We can write it in more clean way after protonation
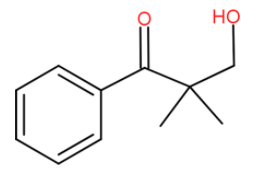
Now we have a carbonyl compound with no α-hydrogen. Now this could be a cross aldol condensation. If this is heated with formaldehyde. If we remember cross disproportionation of formaldehyde, it typically gets oxidised to HCOO−and the carbonyl compound reduces.
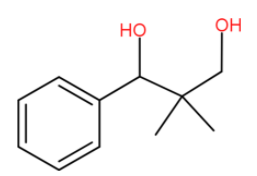
Hence the ketone group reduces to alcohol.
Now this is the consequence of the first reaction. We have completed only the first reaction. We should note that OH− is doing double duty first because it is a quick reaction and once aldol is not possible and we keep heating we get a disproportionation product also.
(ii) Now we are adding more formaldehyde in acidic medium (catalytic amount). Now the product we got from the first reaction with formaldehyde we can clearly see a formation of acetal.
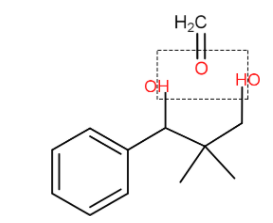
In formaldehyde the CH2O−will combine the two hydrogens from alcohol group and release it as water forming the acetal hence we get acetal

**Therefore, option (A) is the correct option.
Note:**
We can also check the options to find it more conveniently. We may get mistaken for choosing (b) or (c). (b) and (c) are hemiacetals. Hemiacetal means a single carbon attached to -OH group as well as -OR group is known as a hemiacetal group. Also hemiacetals are typically unstable and get decomposed. Also (d) cannot form since the aryl group never gets involved in the reaction, only the functional group is affected.
