Question
Question: The major product of the following reaction is...
The major product of the following reaction is
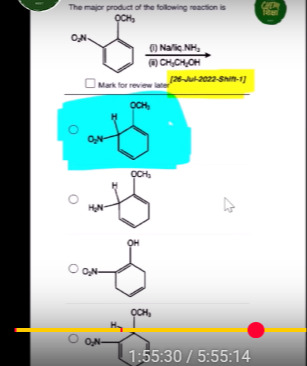
O2N OCH3
H2N OCH3
O2N OH
O2N OCH3
4
Solution
The reaction is a Birch reduction. Sodium in liquid ammonia reduces the aromatic ring to a 1,4-cyclohexadiene. The nitro group is an electron-withdrawing group, and the methoxy group is an electron-donating group. The reduction occurs at positions ortho and para to the electron-withdrawing group and ortho and para to the electron-donating group. In this case, the nitro group directs the reduction to the para position, and the methoxy group also influences the regioselectivity. The major product is the 1,4-cyclohexadiene formed by saturation at the positions para to the nitro group and ortho to the methoxy group, with the double bonds remaining in conjugation. The subsequent protonation with ethanol completes the reaction.
