Question
Question: The major product of the following reaction is: 
(A)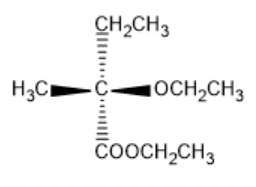
(B)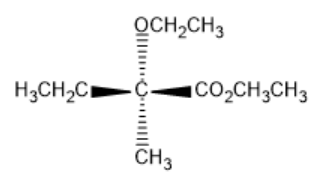
(C)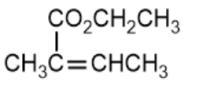
(D)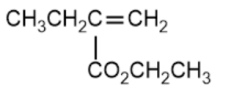
Solution
Hint : When a reaction takes place in the presence of sodium ethoxide which is a strong base, we get two alkenes as products. The products formed follow Zaitsev's rule. The trans product is the major product and the cis product is the minor product. This is due to less steric crowding in the trans product.
Complete Step By Step Answer:
In organic chemistry, Sodium ethoxide is a strong base and is used in reactions such as deprotonation, dehydration and dehalogenation. The OEt− present in sodium ethoxide extracts the hydrogen present in the given compound. The hydrogen leaves as H+ and this leaves a negative charge on the carbon which donated the hydrogen atom. This will cause the chlorine atom which is more electronegative to leave as chloride ion. Due to the difference in electron densities of both adjacent carbon atoms, a double bond is formed between them. This reaction is an elimination reaction and as the two atoms attached to adjacent carbon atoms are removed, a double bond is formed.
The reaction takes place as follows:
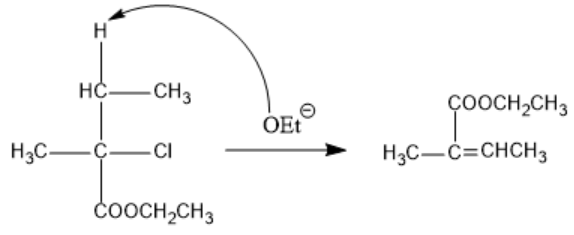
Note :
Sodium ethoxide helps in formation of alkenes as products in a chemical reaction. It will help in the formation of a double bond on the carbon that is most substituted. The heat helps in increasing the temperature so that the reaction will take place easily. At higher temperatures, the reaction rate is usually faster as the molecules move faster and form the products.
