Question
Question: The major product of the following reaction is: 
A.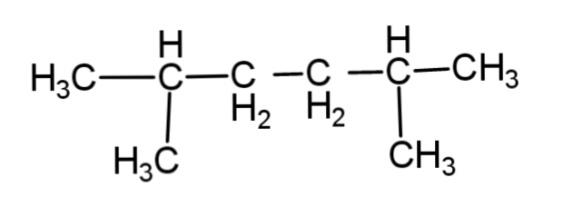
B.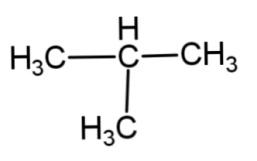
C.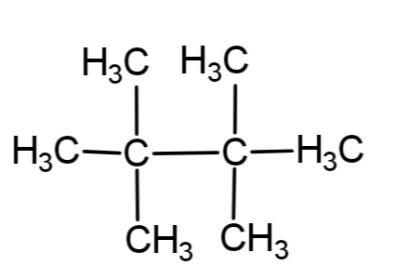
D.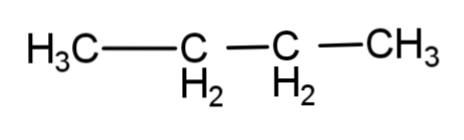
Solution
When an alkyl hydrocarbon chain is attached with a halogen then they are termed as alkyl halides. These alkyl halides can be used in synthesis of higher alkyl chain hydrocarbons. The presence of a catalyst as metallic sodium and dry ether results in a reaction called Wurtz reaction, where alkyl halides are reactants that form higher alkanes.
Complete answer:
We have been given a reaction equation where a tertiary alkyl halide reacts with metallic sodium in the presence of dry ether to form a product. This type of reactions where alkyl halides react in presence of sodium and dry ether are called wurtz reactions.
The reaction involves the formation of a higher alkane with double the number of alkyl groups as in the reactant. This is applicable only till the primary and secondary alkyl halides. Tertiary alkyl halides do not associate to form higher alkane, rather they form a simple alkane as a major product and alkene as minor product by dehalogenation.
Hence, the major product of the following reaction is as follows:
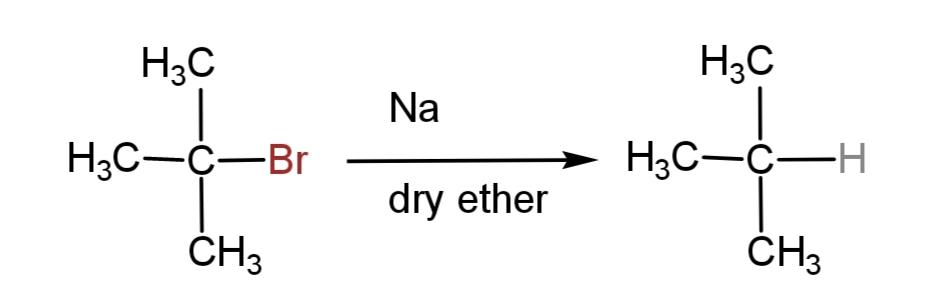
So, option B is correct.
Note:
The wurtz reaction can form only an even number of hydrocarbons. So, it is applicable to the reaction of symmetrical alkyl halides only and does not include the reaction of tertiary alkyl halides due to large amounts of steric hindrance. The condition dry ether is used as sodium is a highly reactive metal that can even react in moisture, so dry ether is used as a solvent to carry out the reaction in a dry environment.
