Question
Question: The major product of the following reaction is: 
(a)- 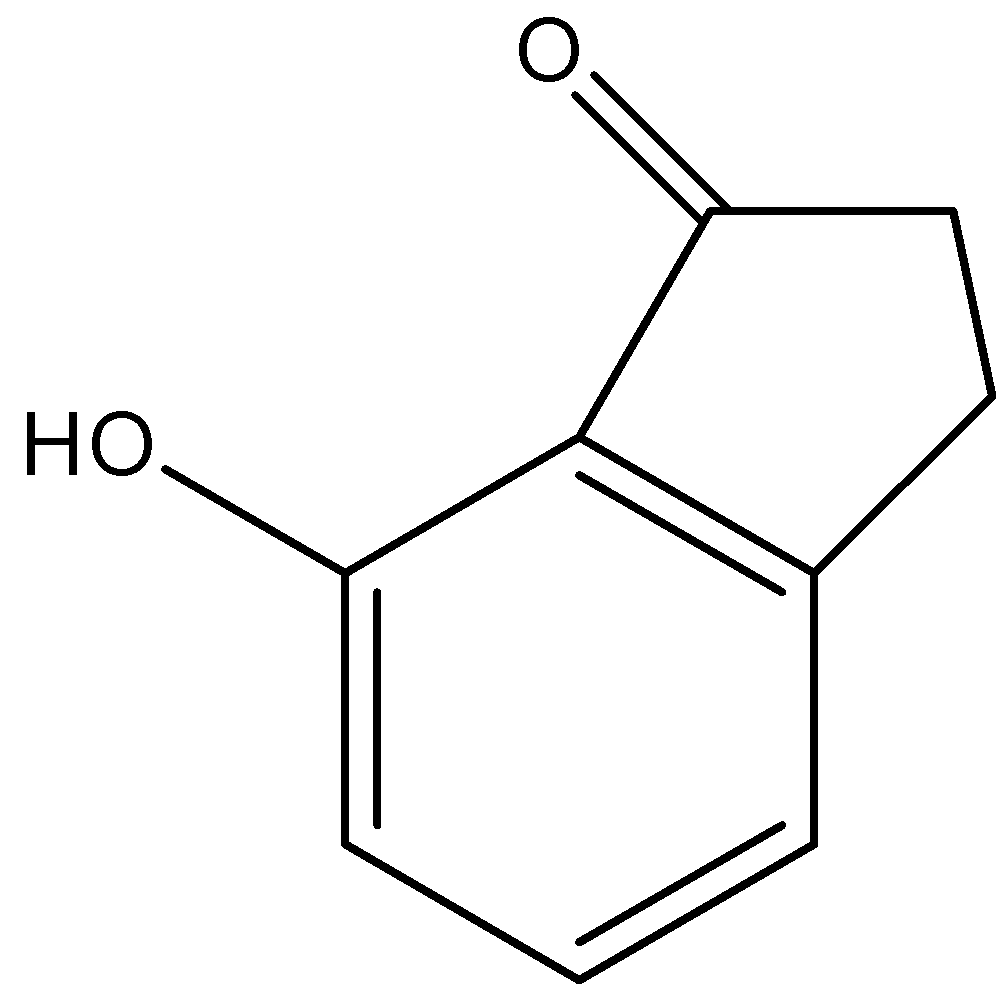
(b)- 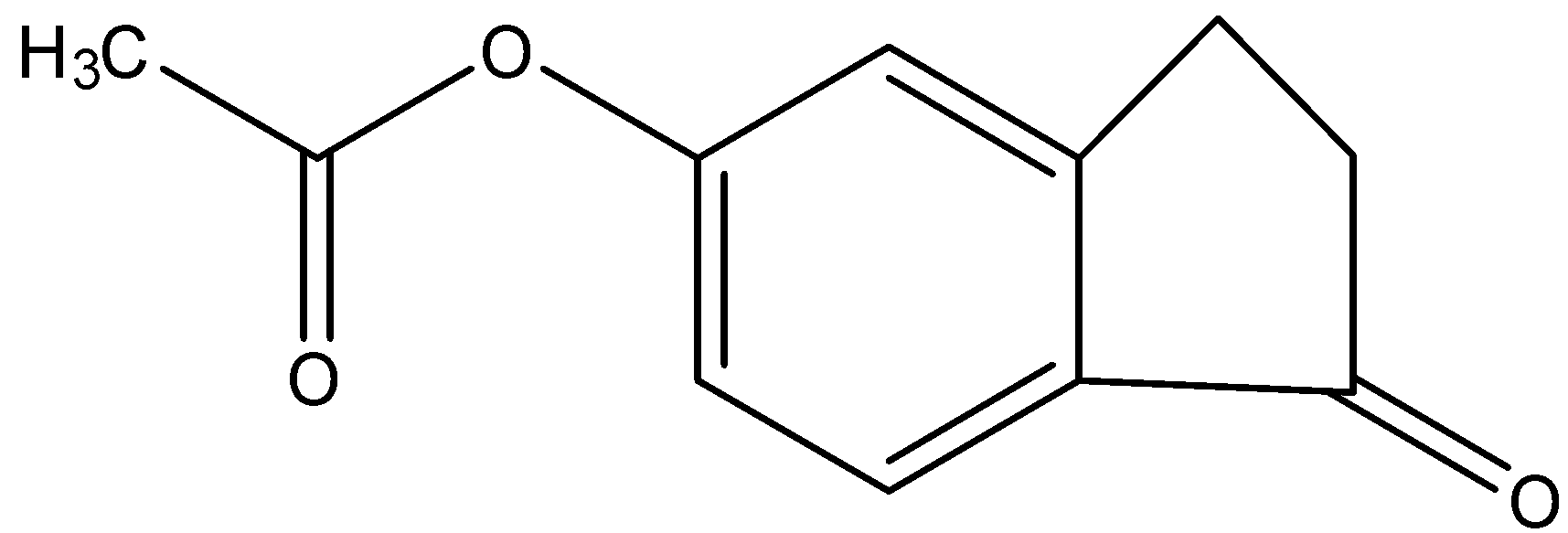
(c)- 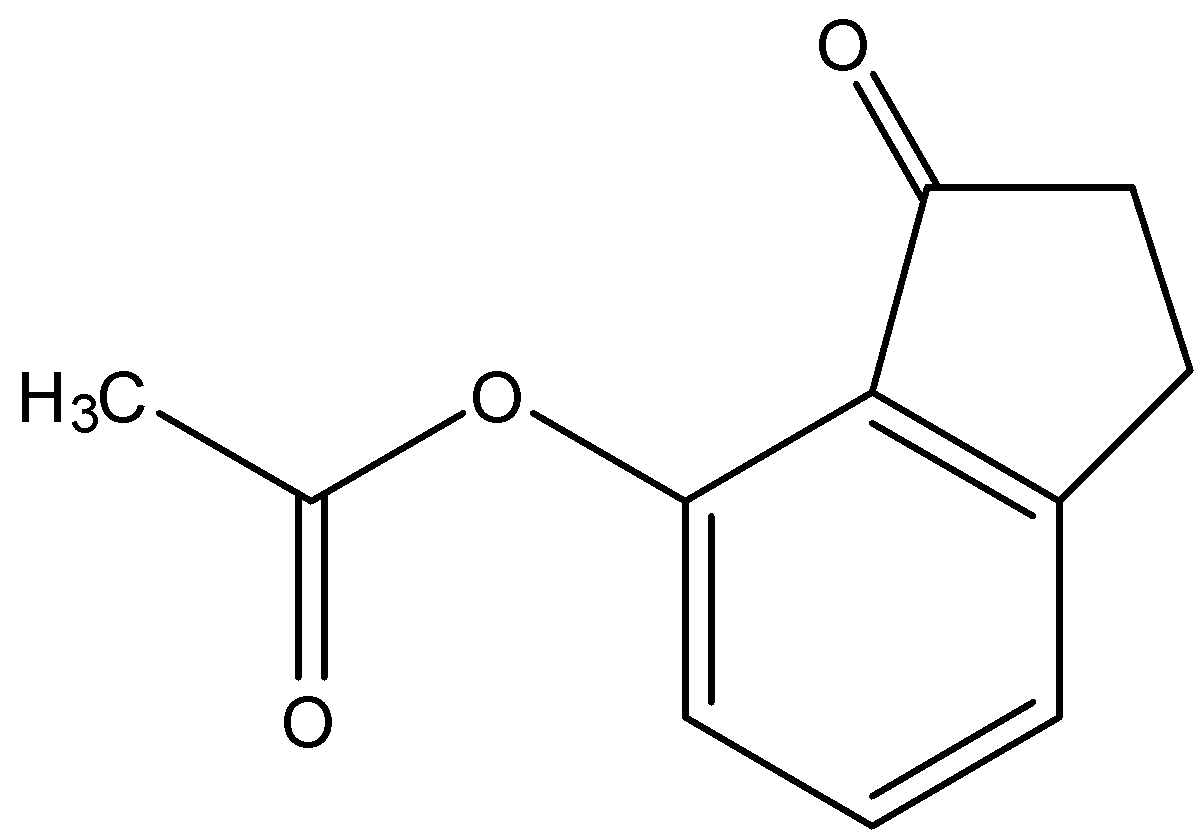
(d)- 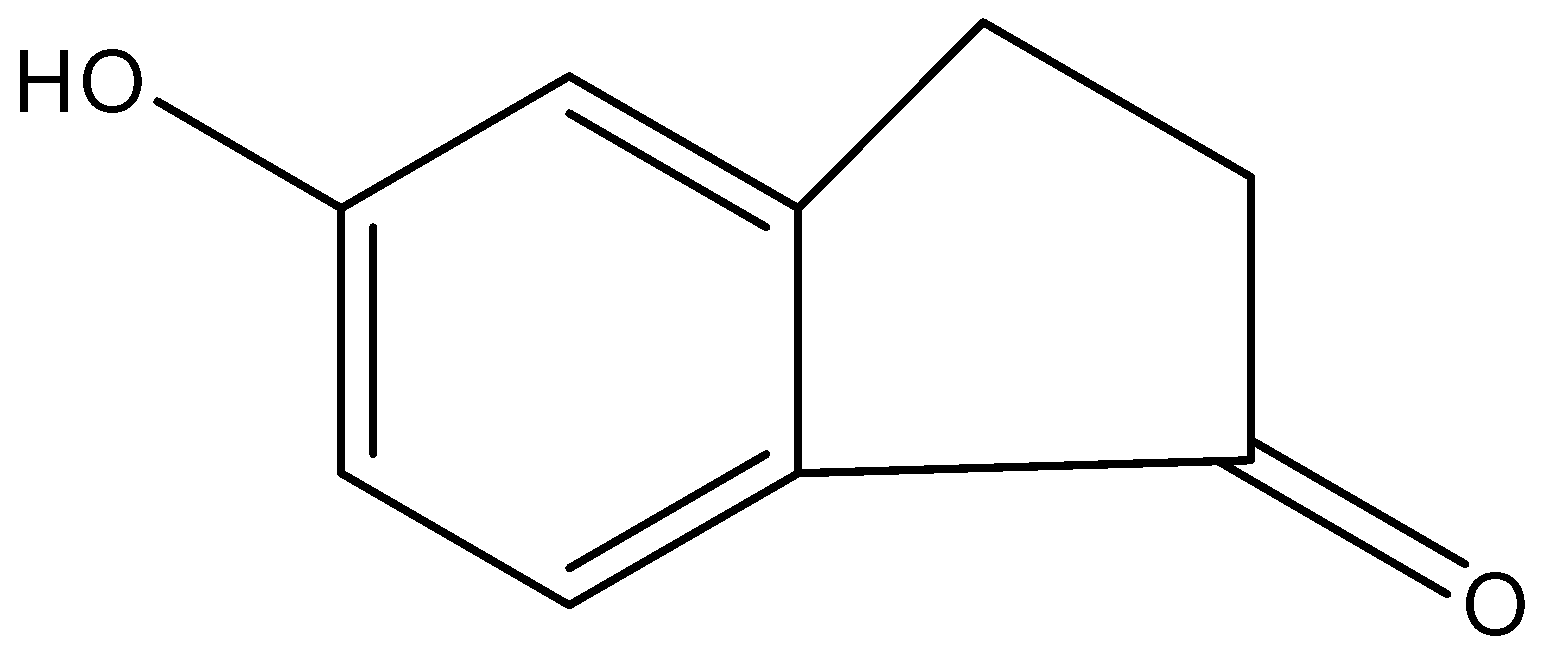
Solution
The sodium nitrite in the presence of acid will convert the amine group in the compound to the hydroxyl or alcohol group. Chromium carbonate in the presence of acid is an oxidizing agent which will oxidize the hydroxyl group. Sulfuric acid will convert the straight chain into cyclic form.
Complete answer:
The given reactant in the reaction is an aromatic compound in which an aliphatic amine group is present and an acetoxy group is also present. The reactant is treated with three catalysts in the reaction.
The first catalyst in the reaction is sodium nitrite (NaNO2) in the presence of acid. So, when the reactant reacts with this catalyst then, the amine group in the reactant will convert into a hydroxyl group. The reaction is given below:
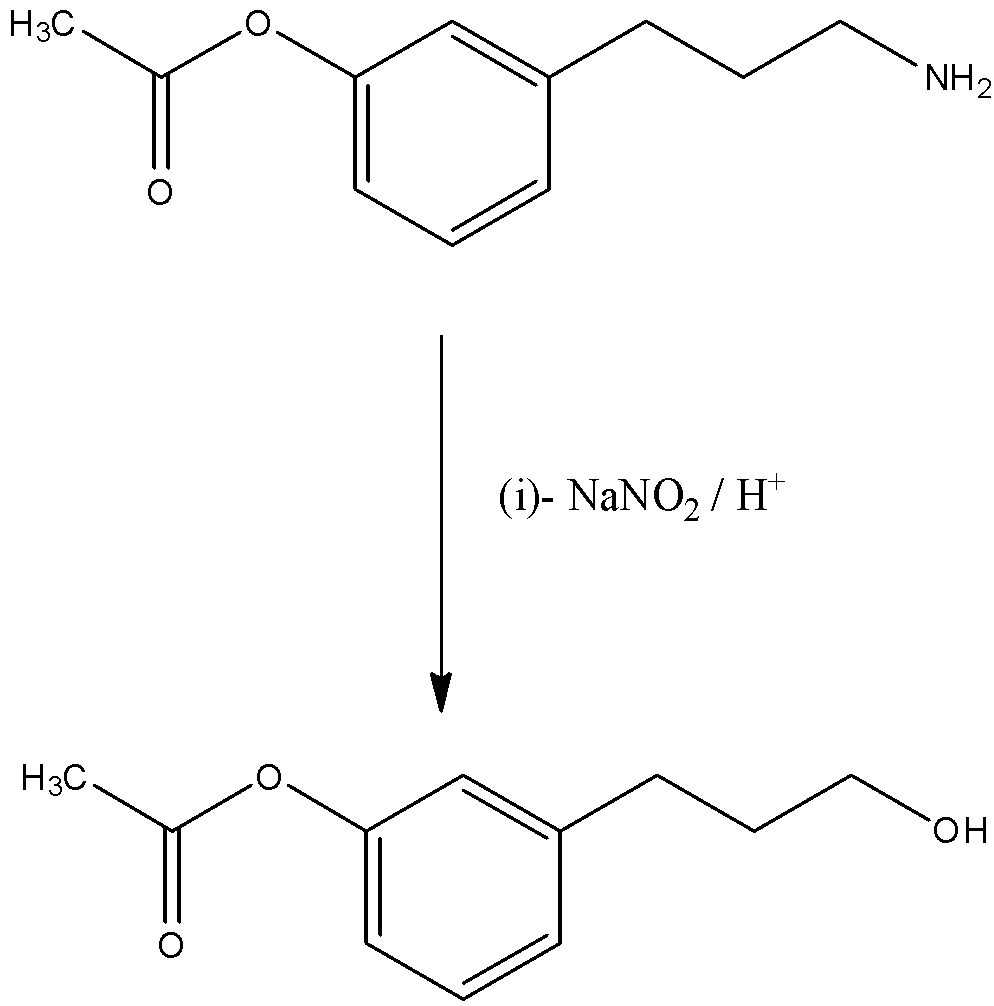
The second catalyst is chromium carbonate (CrCO3) in the presence of acid and this is a strong oxidizing agent so, the hydroxyl group in the reactant will be directly converted into a carboxylic acid group. The reaction is given below:
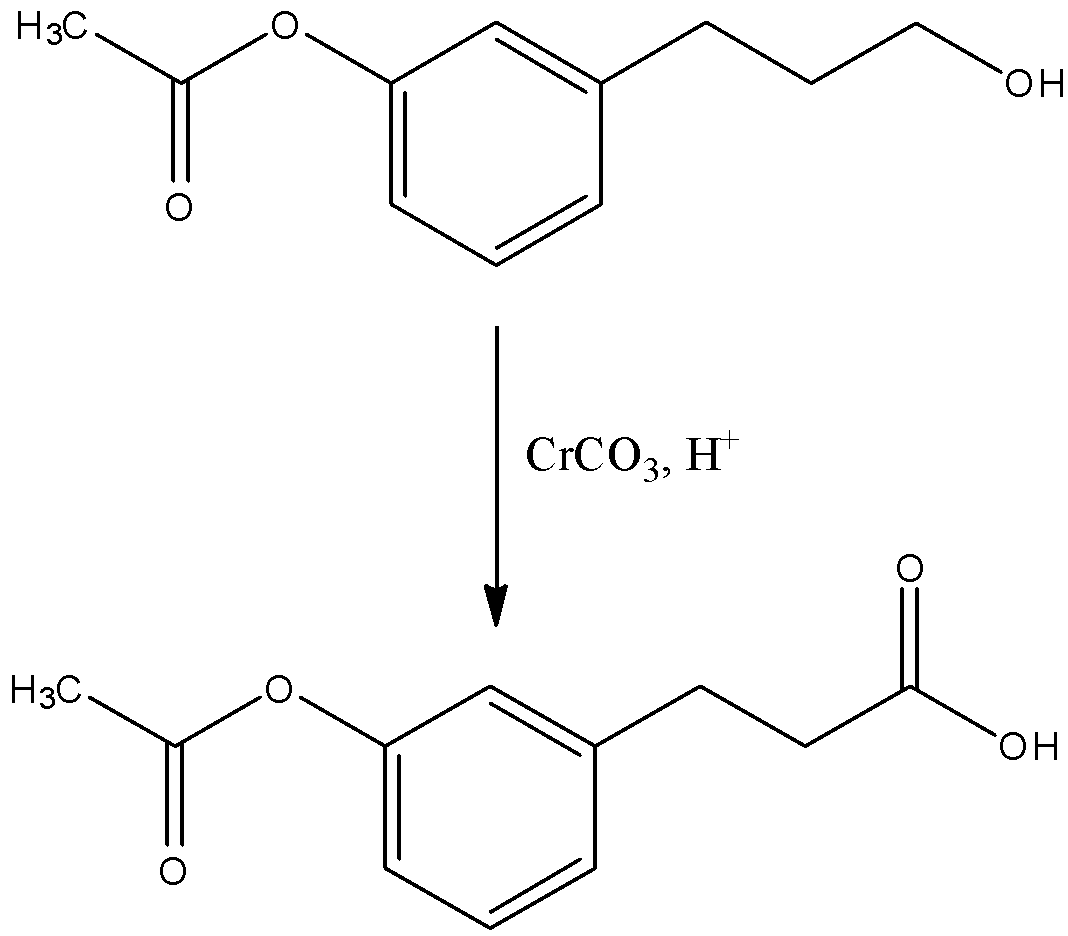
The third catalyst is concentrated sulphuric acid. When the reaction with the compound then the chain having the carboxylic acid will convert into a cyclic chain with the next carbon atom in the aromatic ring to which the chain is attached. So, there are two positions in the aromatic ring, therefore, the ring will be formed which will have more stability. The reaction is given below:
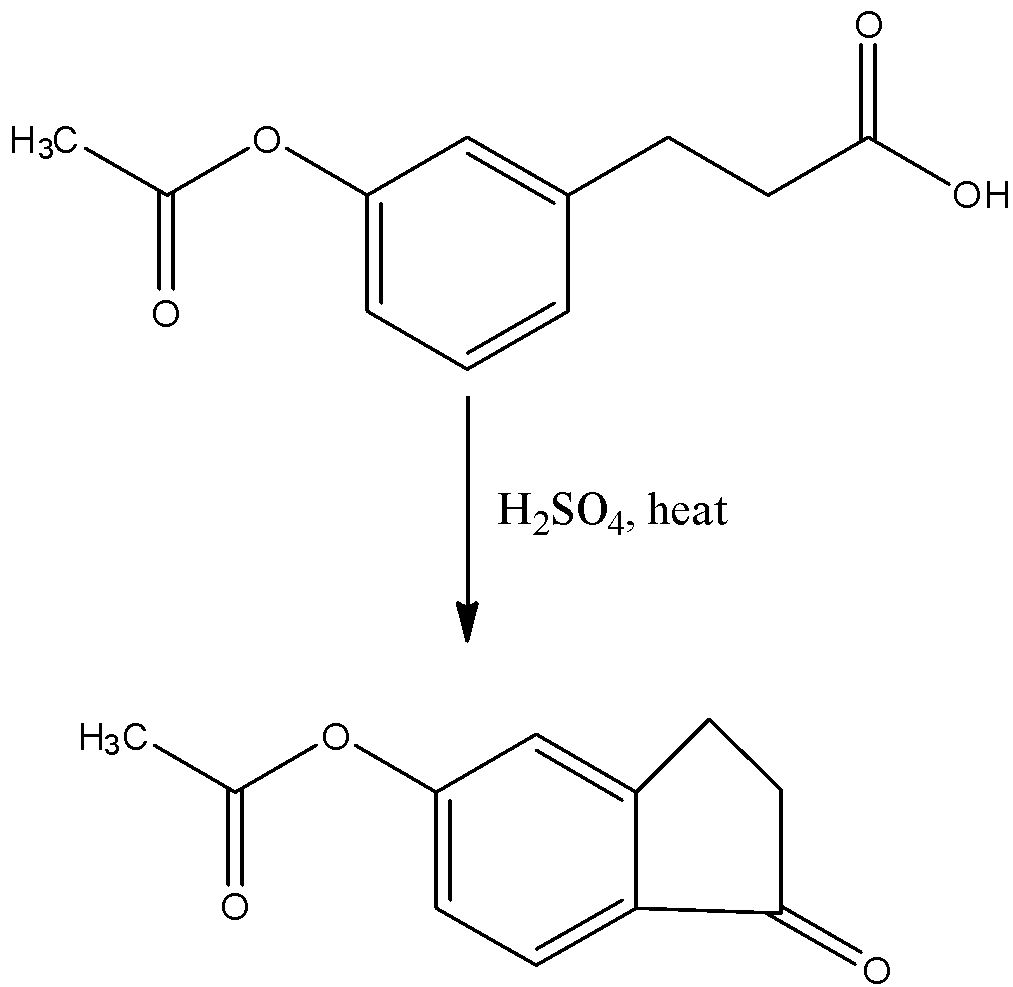
Therefore, the correct answer is an option (b).
Note:
If the cyclic chain is formed on the ortho position of the acetoxy group then the stability will decrease because there will be a steric hindrance. Chromium carbonate is used to oxidize the compound directly to carboxylic acid.
