Question
Question: The major product of the following reaction is 
A.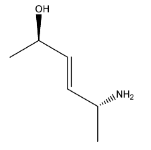
B.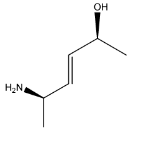
C.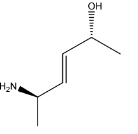
D.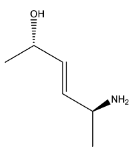
Solution
As we know that when alkyl halide reacts with aqueous KOH (potassium hydroxide), the hydroxide ion of KOH reacts as a nucleophile and replaces the halogen from alkyl halide to give us alcohol. This process occurs through SN2 Reaction Mechanism. We considered it as aqueous KOH because options have substituted product of alkyl halide by hydroxide ion instead of elimination product.
Complete step by step answer:
As it is given in the question, the reaction will proceed through SN2 Mechanism.
- SN2 Reaction Mechanism involves the attack of nucleophile from the opposite side of the leaving group attached to the carbon atom. So the product assumes a stereochemical position opposite to the position originally occupied by the leaving group. Also, SN2 Reaction Mechanism is the most common example of Walden inversion where an asymmetric carbon atom undergoes inversion of configuration.
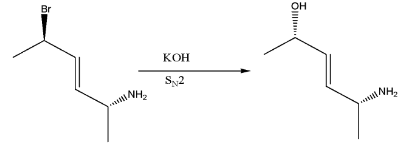
-The aqueous KOH is a solution of dissociated ions shown below:-
KOH⇌K++OH−
-This hydroxide ion acts as a strong nucleophile and when it reacts with alkyl halide, it replaces the halogen group from it through SN2Reaction Mechanism. The product formed will have inversion of configuration at the site of reaction.
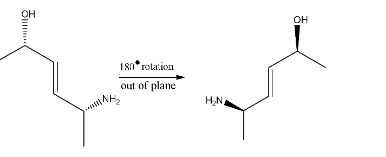
This is the product obtained on reaction with aqueous KOH. But, since it does not match with the options, therefore we will make a 180∘ rotation out of the plane as shown below:-
Hence the major product is Option (B).
Note:
- Aqueous KOH is generally used for the reactions which are not water sensitive and for performing hydrolysis whereas alcoholic KOH is used for the reactions which are water sensitive and for performing dehydration reactions.
- SN2 Reaction Mechanism is a good example of stereospecific reaction.
- Always remember that we can rotate a compound in Wedge projection by 180∘(out of plane) but we cannot do the same for Fischer projection.
