Question
Question: The major product of the following reaction is: 
A.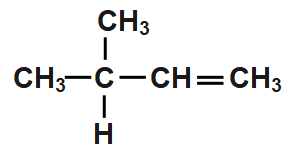
B.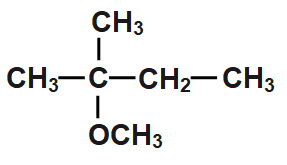
C. 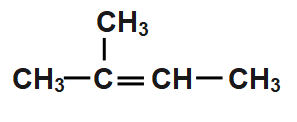
D. 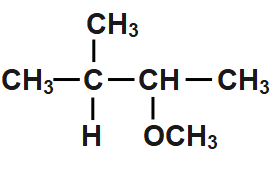
Solution
To solve this we can use the Saytzeff’s rule. Saytzeff’s rule is used in the analysis of elimination reactions. The elimination reactions of alcohols and halides produce alkenes. In elimination reactions, the major product is obtained by the Saytzeff’s rule.
Complete step by step solution:
We are given CH3CH(CH3)CH(Br)CH3 which is treated with CH3OH. The product of this reaction is obtained by Saytzeff’s rule. According to Saytzeff's rule, the major alkene is formed by the removal of hydrogen from a -carbon which has a low number of substituents.
When CH3CH(CH3)CH(Br)CH3is treated with in it undergoes dehydrobromination reaction. in the dehydrobromination reaction, a hydrogen bromide molecule is eliminated.
According to Saytzeff’s rule, the major alkene is formed by the removal of hydrogen from α−carbon which has a low number of substituents. In the dehydrobromination reaction, a hydrogen bromide molecule is eliminated.
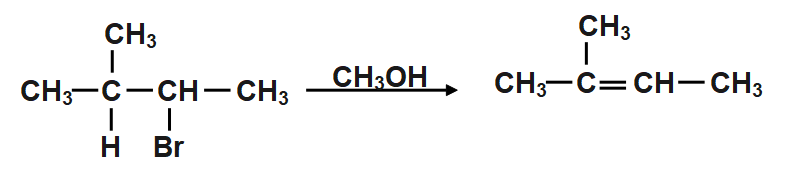
According to the Saytzeff’s rule, CH3CH(CH3)CH(Br)CH3undergoes dehydrobromination and hydrogen bromide gets eliminated from CH3CH(CH3)CH(Br)CH3is formed as the major product. Thus, when CH3CH(CH3)CH(Br)CH3is treated with in , the product formed is option c
So, the correct answer is Option C.
Note: We have solved the given reaction of CH3CH(CH3)CH(Br)CH3 within CH3OH, with the help of Saytzeff’s rule. We know that the saytzeff’s rule favors the alkene with less number of hydrogen on double bonded carbon atoms. In the reaction dehydrobromination of CH3CH(CH3)CH(Br)CH3 occurs. Thus, Saytzeff’s rule is applicable in this case. During an elimination reaction, a proton is removed from the carbon atom having less number of substituents.
