Question
Question: The major product of the following reaction is: 
A.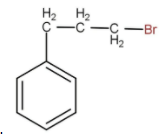
B.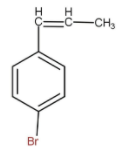
C.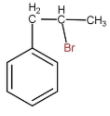
D.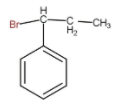
Solution
The above reaction is the additional reaction. The molecule hydrogen bromide that is HBr, adds up to the unsaturated carbon according to markovnikov addition. Carbocation will form as an intermediate during the reaction
Complete step by step answer:
The addition of HBr to alkene involves the formation of free radicals as the intermediate. More are the number of carbon attached with free radical; more stable is the free radical due to the positive inductive effect of the alkyl group. Hence tertiary free radical is more stable than secondary free radical and primary free radical is least stable.
The name of the reactant given is 1-phenylpropene.
The reaction follows markovnikov addition; it states that in an unsymmetrical alkenes addition will occur on the more substituted carbon or the carbon which has less number of hydrogen. Both of the carbon in alkene are equally substituted and hence have the same number of alpha hydrogens.
In the above question if carbocation forms on carbon number 1, then it will be stabilized by the resonance effect of pi electrons of the phenyl group and hence is more stable. If the carbocation forms on carbon number 2, then it will only be stabilized by hyperconjugation. Resonance effect is prioritized over the hyperconjugation. So the product forms is
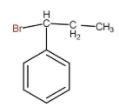
Hence, the correct option is D.
Note:
Alkenes are unsaturated hydrocarbons containing at least one double bond. Unsaturated hydrocarbons are those hydrocarbons which contain double bond or triple bond. The name of the reactant used above is phenylpropene and exists in liquid state.
