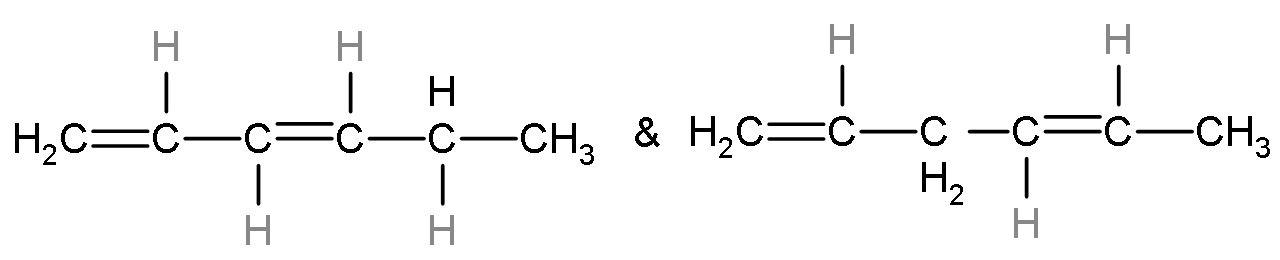Question
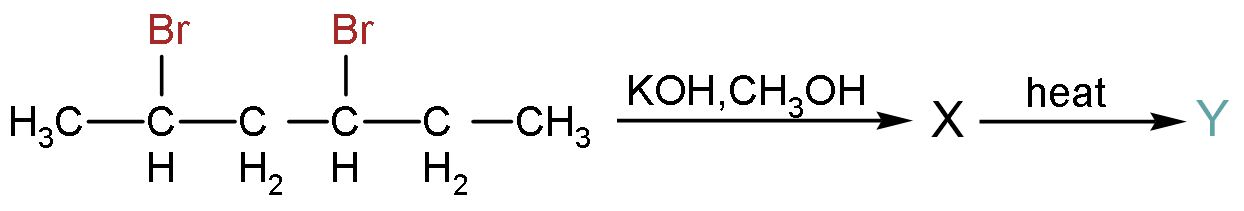
Question: The major product of the following reaction is: 
Solution
The above reaction follows elimination. This reaction is an organic reaction in which two substituents are removed in either a one or two-step mechanism. The alkenes are formed. Follow the mechanism and obtain the answer. Take the reagent one-by-one and react with the reactant.
Complete step by step answer:
Let us obtain the final product by two steps, one addition of (−OH) group and other is elimination of (−OH) group to form alkenes. This is the one reaction only, but we will do this reaction two steps to understand it in a better manner.
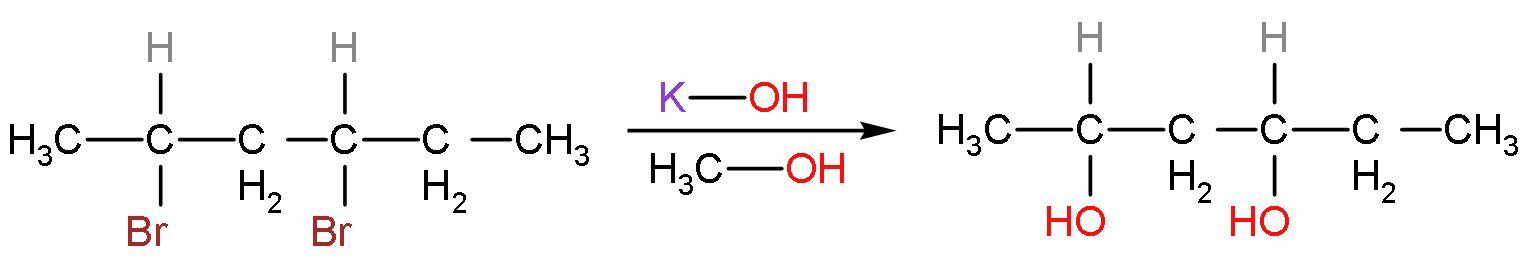
Step (1)- The hydroxide ions from the base potassium hydroxide (KOH) in presence of methyl alcohol. The Br− ions are replaced by the OH− ions to form the required product. The product formed is
Step (2)- Now, the product of the previous step is heated, it means the compound will be dehydrated, to release water and form the final product (alkenes). Carbocation is formed as an intermediate. The alkenes will be formed according to Saytzeff’ rule. This rule states that, ‘alkene product formed will be major if that alkene corresponds to the removal of hydrogen atoms from the adjacent carbon having the fewest hydrogen substituents’.
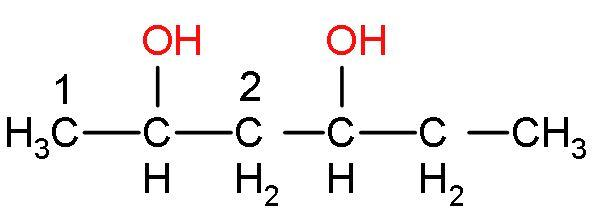
in this compound, carbon 1 has more hydrogen than carbon 2. Similarly, the other OH− group will also be eliminated like this only. The compound formed will be a conjugated system, so it will undergo resonance. This reaction is an elimination reaction or E2 reaction. So, the alkene formed will be
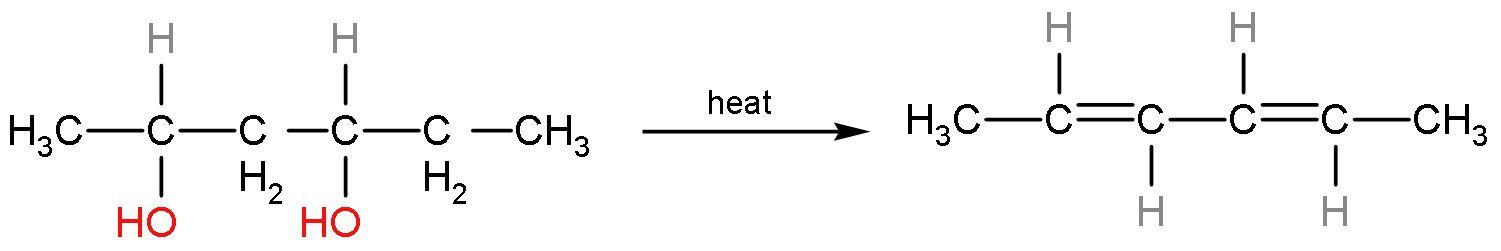
The IUPAC name of the product is penta-2,4-diene. The alkene formed is stable as it has six hyperconjugating structures. The count of alpha hydrogen atoms is the count of hyperconjugating structures.
Note: The minor product of the reaction will be the product not favoured by Saytzeff’ rule. So, the percentage of minor products will be less. The minor products are