Question
Question: The major product of the following reaction is: 
Solution
We will first identify the functional group in the reactant and the group that will react with the given reagents. The functional group will not be altered because of the reagents due to the resonance effect.
Complete solution:
We can see that the OH group is the functional group in the reactant, and is directly bonded with the benzene group, this direct bonding will cause resonance and the C−O will become stronger, and hence there will be no direct reaction of the given reagents and the functional group (OH).
However, the butene (or butylene) group bonded at the meta position will react with the HCl, and anhydrous AlCl3 to yield a product following Markovnikov’s rule. The reaction and be written as:
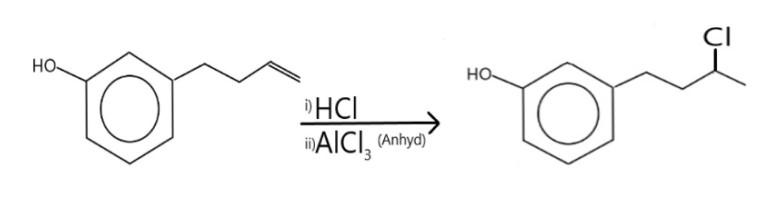
Additional information:
According to Markovnikov’s rule, the negative part of an H−X(X=Cl,Br,I) will get bonded to the carbon having less number of hydrogen.
However, the above condition can be altered and the reaction can be altered by adding peroxide. When peroxide is added to the reaction mixture the negative part will make a bond with the carbon having more hydrogen.
Note: It is possible to form aryl halides from aliphatic alcohol, but with phenols, it is not possible because of the resonance effect, and due to this effect the C−O bond becomes C=O in some resonating structures, this double bond is stronger than the single bond and hence cleavage of C−O is not possible in phenols.
