Question
Question: The major product of the following reaction is: 
(A) 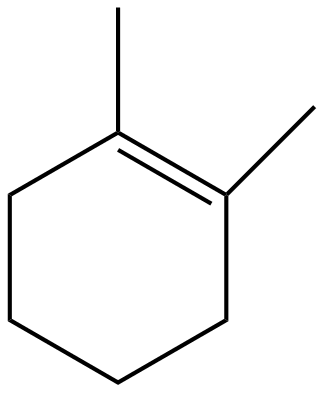
(B) 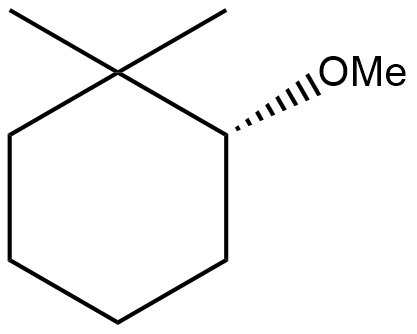
(C) 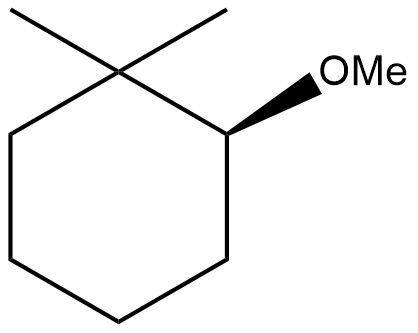
(D) 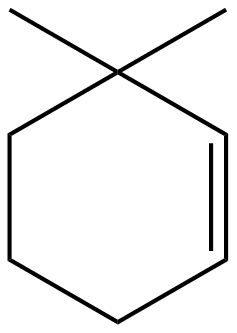
Solution
The given reaction will be the dehydrohalogenation reaction. As the name suggests, this would be typically an elimination reaction with elimination of a hydrogen and a halogen from the given molecule forming an alkene.
Complete answer:
Let us see the reaction type and mechanism in detail before solving the given reaction;
Dehydrohalogenation-
This is the elimination reaction that eliminates or removes a hydrogen and a halide i.e. in the form of hydrogen halide from the substrate. The mechanism of this reaction is simple as it is directly associated with the synthesis of alkenes. This reaction has a wide range of applications.
Generally, this reaction takes place in the presence of base; this reaction is known as β−elimination which is a type of elimination reaction. For example;
CH3CH2CH2Cl+KOH→CH3CH=CH2+KCl+H2O
So, concentrating on our given reaction;
The bromide ion and the hydrogen will leave the molecule in the form of hydrogen bromide forming an alkene. Thus, forming the molecule as in option (D). In the way
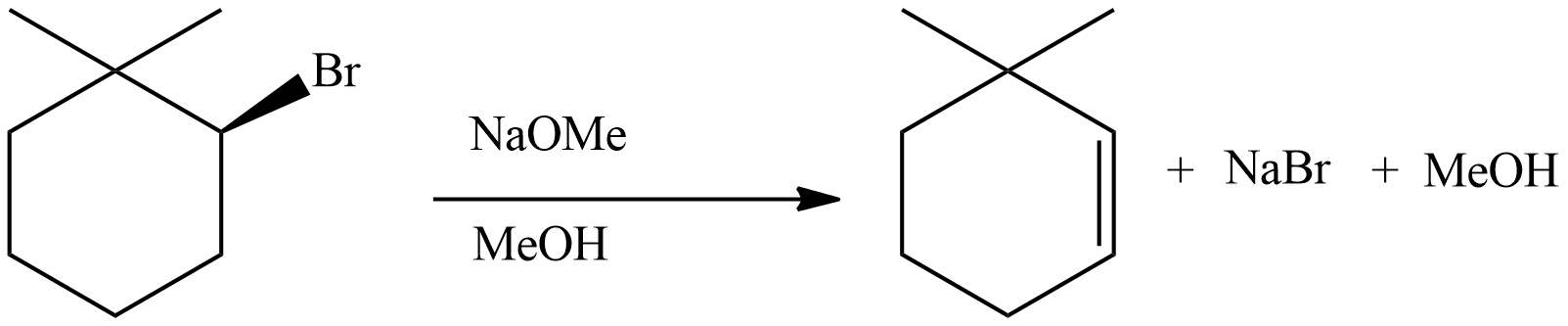
From this, we can say that the reaction here is associated with the formation of an alkene which is possible due to the removal of a hydrogen and a halide group from the given molecule.
Option D is the correct answer.
Note:
Do note that the reaction is completely elimination reaction which results into the formation of alkene; the aim of dehydration or dehalogenation or dehydrohalogenation is same i.e., elimination with formation of a new molecule (mostly alkenes and alkynes). So, option (A), (B) and (C) will never form as such.
