Question
Question: The major product of the following reaction is: - 
A.
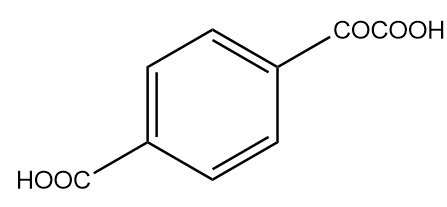
B.
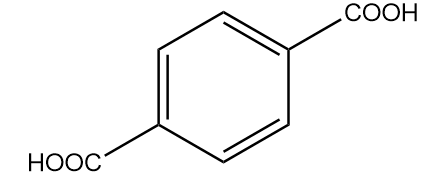
C.
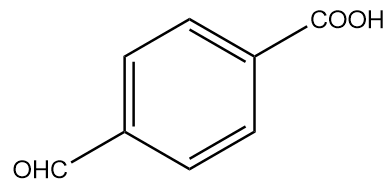
D.
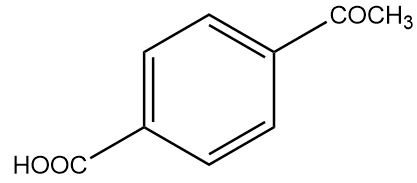
Solution
In an organic reaction, if a compound gains an electronegative element or loses an electropositive element then the reaction is known as oxidation reaction whereas if the compound loses an electronegative element or gains an electropositive then the reaction is known as reduction reaction.
Complete answer:
For the given reaction sequence, the reaction mechanism proceeds as follows:
Step-1: The methyl group and acetyl group react in the presence of potassium permanganate, then both the groups get oxidized to the carboxyl group. The reaction proceeds as follows:
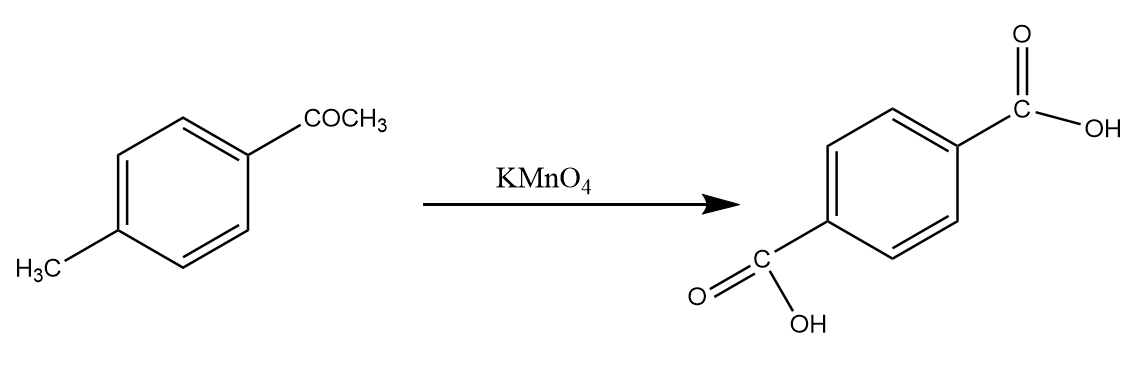
Step-2: Potassium hydroxide is a good base and has a tendency to extract protons from the acidic functional groups. Therefore, when carboxyl groups react with potassium hydroxide in the presence of heat, the group releases its proton to form the potassium salt of the compound. The reaction proceed as follows:
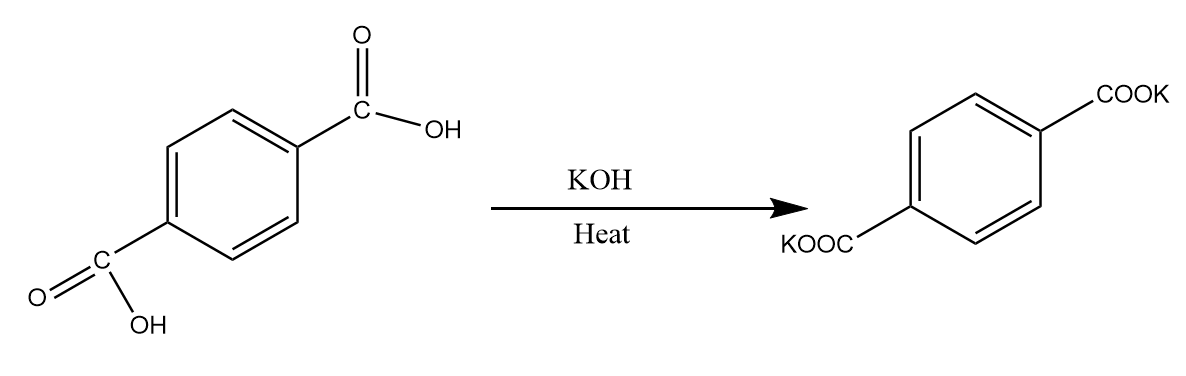
Step-3: When the potassium salt of the compound is reacted with sulphuric acid that means, a source of protons is being provided to the compound. Therefore, the potassium salt of acid gets converted back onto carboxyl groups. The reaction takes place as follows:
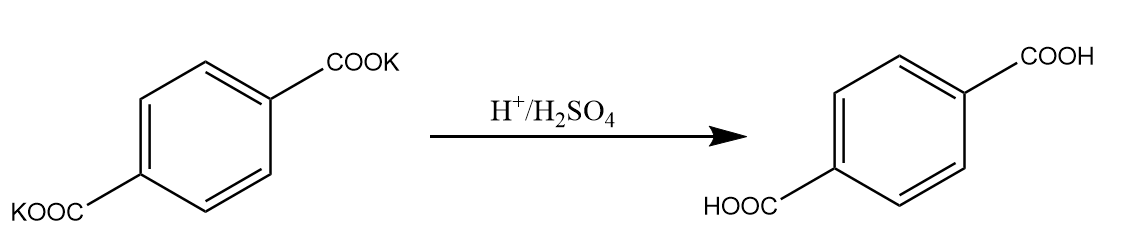
Hence, the major product formed in the reaction is terephthalic acid.
Thus, option (B) is the correct answer.
Note:
It is important to note that the oxidation reaction in the presence of potassium permanganate will work only if there is at least one hydrogen atom attached to the alkyl group. If in case there is no hydrogen atom present, then no reaction will take place.
