Question
Question: The major product of the following reaction is: 
(a)
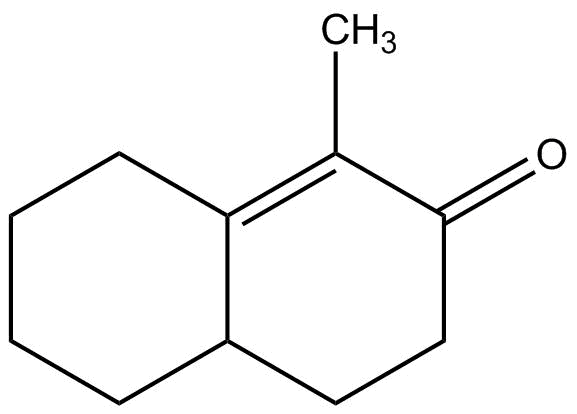
(b)
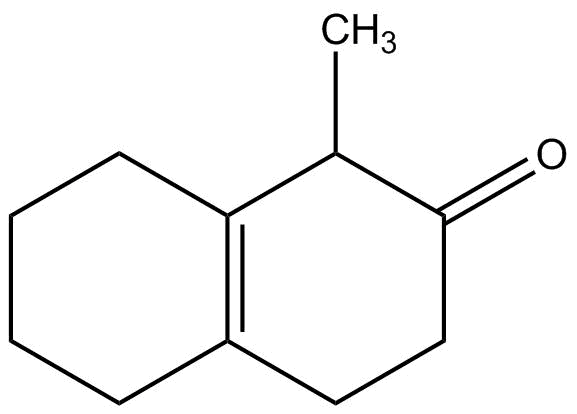
(c)
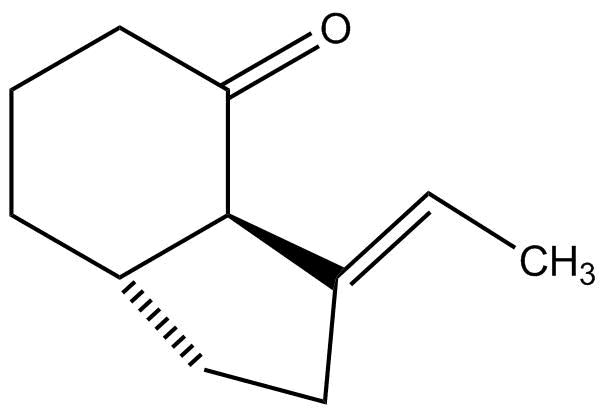
(d)
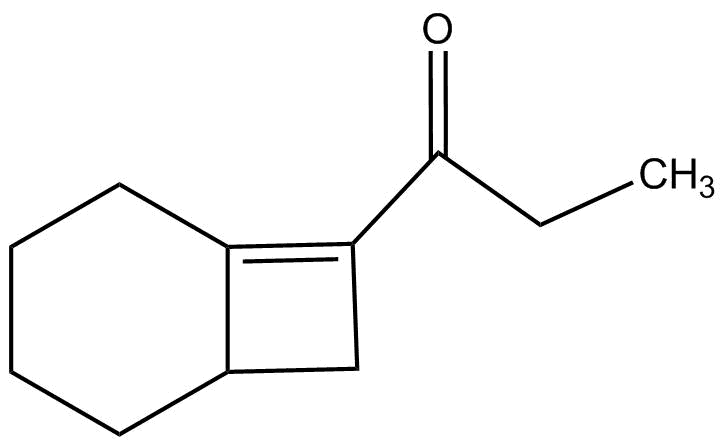
Solution
Aldol condensation is a reaction under organic chemistry in which an enol or an enolate ion reacts with a carbonyl compound to form a β− hydroxy− aldehyde or β− hydroxy ketone, followed by dehydration to give a conjugated enone.
Complete step by step answer:
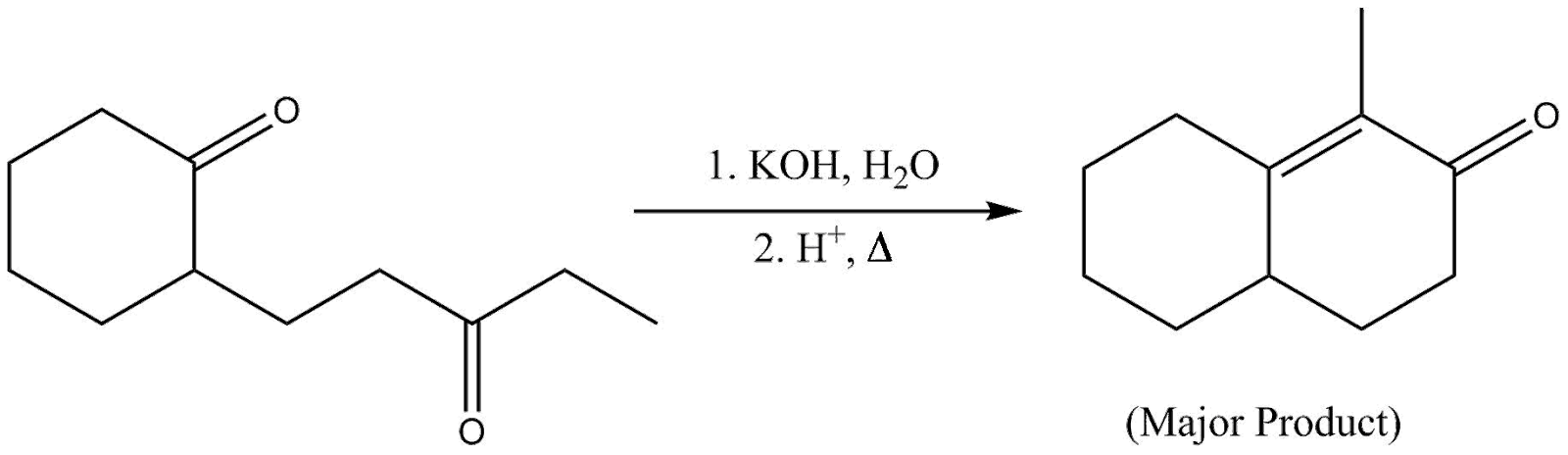
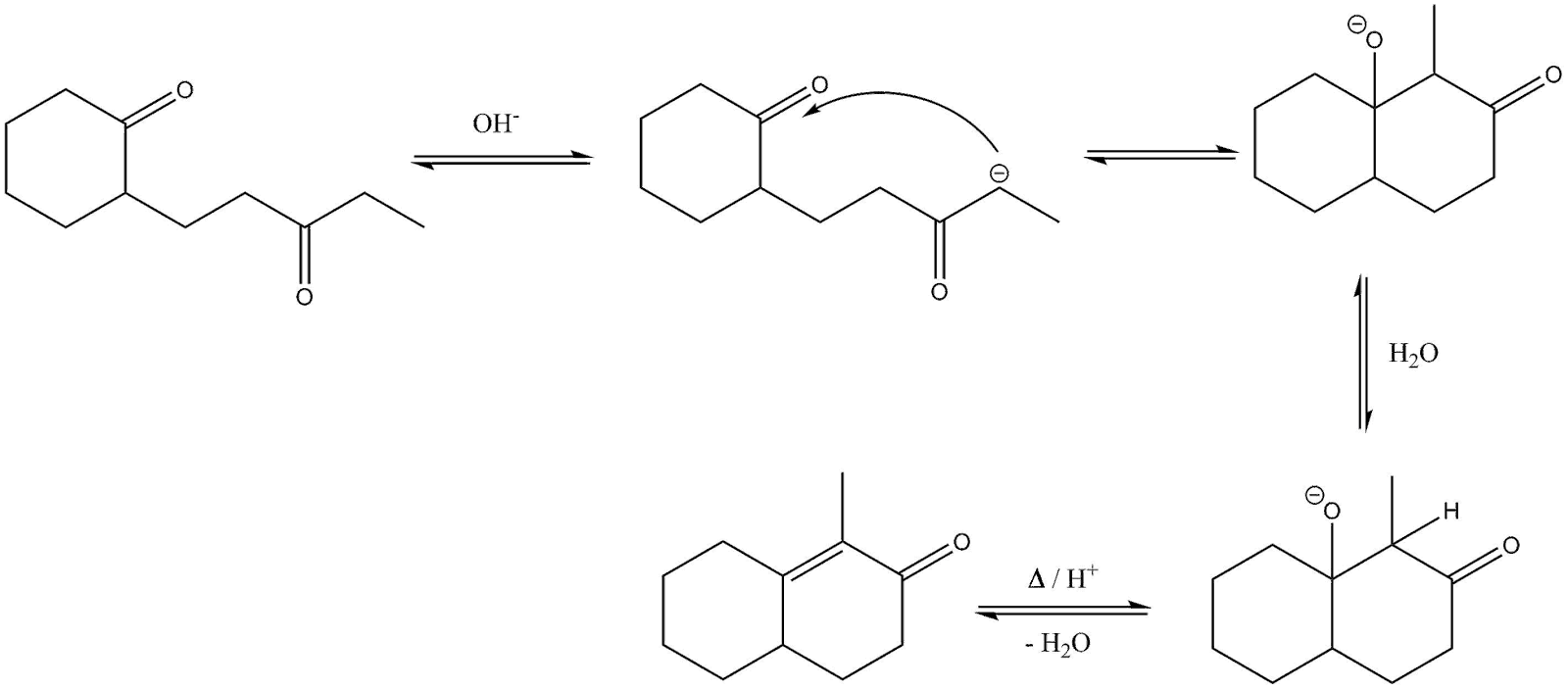
Mechanism:
Additional Information:
(1) Aldols are easily dehydrated to α,β− unsaturated compounds when heating alone or with acid or base.
(2) When aldol condensation reaction is carried out in the presence of strong alkali, repeated condensation and dehydration results in the formation of resins.
(3) The aldol condensation is promoted by the−I effect and reduced by +I effect on the carbonyl carbon.
(4) The reaction equilibrium is much favorable for aldehydes less favourable for ketones.
(5) When aldehydes and ketones both having α−hydrogens are condensed, two products are obtained. This is because ketones are poor carbanion acceptors and cannot undergo self condensation. Aldehydes being more reactive than ketones act as carbanion acceptors and the ketones provide the carbanions.
So, the correct answer is Option A.
Note: The aldol condensation reaction was discovered by the Russian Chemist Alexander Borodin in 1869 and by the French chemist Charles-Adolphe Wurtz in 1872 The reaction is about combining two carbonyl compounds to form a new β− hydroxyl carbonyl compound.
